- Home
- About Journals
-
Information for Authors/ReviewersEditorial Policies
Publication Fee
Publication Cycle - Process Flowchart
Online Manuscript Submission and Tracking System
Publishing Ethics and Rectitude
Authorship
Author Benefits
Reviewer Guidelines
Guest Editor Guidelines
Peer Review Workflow
Quick Track Option
Copyediting Services
Bentham Open Membership
Bentham Open Advisory Board
Archiving Policies
Fabricating and Stating False Information
Post Publication Discussions and Corrections
Editorial Management
Advertise With Us
Funding Agencies
Rate List
Kudos
General FAQs
Special Fee Waivers and Discounts
- Contact
- Help
- About Us
- Search

The Open Bioactive Compounds Journal
(Discontinued)
ISSN: 1874-8473 ― Volume 9, 2020
Ameliorative Potential of Natural Antioxidants Against Paraquat-Induced Oxidative Stress and Locomotor Impairment in Drosophila melanogaster: A Comparative Study
S. Niveditha, T. Shivanandappa*, S.R Ramesh
Abstract
Background:
Natural antioxidants show neuroprotective potential to protect against neurodegenerative disorders in experimental animals. There is a need to characterize newer promising neuroprotective natural molecules.
Objective:
In the present study, we have compared the neuroprotective activity of 4hydroxyisophthalic acid (DHA-I), a novel natural antioxidant from the roots of Decalepis hamiltonii, with the other natural neuroprotective antioxidants, ellagic acid, quercetin and nicotinamide, against paraquat (PQ) neurotoxicity in D. melanogaster.
Results:
Flies exposed to multiple (sub-lethal) dose of PQ showed movement disorder characteristic of Parkinson’s disease (PD). The four natural antioxidants showed ameliorative effects against PQ neurotoxicity in the sub-acute model as seen in survivability, locomotor activity as well as oxidative stress markers including reactive oxygen species (ROS), lipid peroxidation and the endogenous antioxidant defenses.
Conclusion:
Our study shows that the antioxidant compounds exhibit varying degrees of protection against PQ-induced oxidative stress and neurotoxicity with DHA-I, quercetin, and nicotinamide being the most effective and ellagic acid, the least potent in Drosophila. Our results show that mitochondrial Mn-SOD is a critical target for PQ neurotoxicity and the neuroprotection by the antioxidants involves the attenuation of mitochondrial ROS production and oxidative damage.
Article Information
Identifiers and Pagination:
Year: 2017Volume: 05
First Page: 43
Last Page: 56
Publisher Id: TOBCJ-5-43
DOI: 10.2174/1874847301705010043
Article History:
Received Date: 05/7/2017Revision Received Date: 25/09/2017
Acceptance Date: 26/09/2017
Electronic publication date: 14/11/2017
Collection year: 2017
open-access license: This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International Public License (CC-BY 4.0), a copy of which is available at: https://creativecommons.org/licenses/by/4.0/legalcode. This license permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
* Address correspondence to this author at the Neurobiology Laboratory, Department of Zoology, University of Mysore, Manasagangotri, Mysuru- 570006, Karnataka, India, Tel: +91 9480475885; E-mail: tshivanandappa@yahoo.com
| Open Peer Review Details | |||
|---|---|---|---|
| Manuscript submitted on 05-7-2017 |
Original Manuscript | Ameliorative Potential of Natural Antioxidants Against Paraquat-Induced Oxidative Stress and Locomotor Impairment in Drosophila melanogaster: A Comparative Study | |
1. INTRODUCTION
Oxidative stress occurs when there is an imbalance between the generation of free radicals and cellular antioxidant defenses [1Halliwell B. Free radicals, antioxidants, and human disease: Curiosity, cause, or consequence? Lancet 1994; 344(8924): 721-4.
[http://dx.doi.org/10.1016/S0140-6736(94)92211-X] [PMID: 7915779] ], which is implicated in the pathophysiology of certain neurodegenerative diseases [2Chen X, Guo C, Kong J. Oxidative stress in neurodegenerative diseases. Neural Regen Res 2012; 7(5): 376-85.
[PMID: 25774178] ]. Paraquat (PQ), a widely used herbicide, induces Parkinson’s disease (PD) phenotype in experimental models including laboratory rats, mice and Drosophila [3McCormack AL, Thiruchelvam M, Manning-Bog AB, et al. Environmental risk factors and Parkinson’s disease: Selective degeneration of nigral dopaminergic neurons caused by the herbicide paraquat. Neurobiol Dis 2002; 10(2): 119-27.
[http://dx.doi.org/10.1006/nbdi.2002.0507] [PMID: 12127150] -6Zhang XF, Thompson M, Xu YH. Multifactorial theory applied to the neurotoxicity of paraquat and paraquat-induced mechanisms of developing Parkinson’s disease. Lab Invest 2016; 96(5): 496-507.
[http://dx.doi.org/10.1038/labinvest.2015.161] [PMID: 26829122] ]. PQ can undergo redox cycling wherein, a divalent cation of PQ (PQ2+) is reduced to monocation radical (PQ+), which reacts with the oxygen molecule to give rise to free radical generation [7Fridovich I, Hassan HM. Paraquat and the exacerbation of oxygen toxicity. Trends Biochem Sci 1979; 4: 113-5.
[http://dx.doi.org/10.1016/0968-0004(79)90395-5] ]. Although the mechanisms underlying PQ induced PD are not fully understood, it is believed to be mediated by free radical-induced oxidative stress that causes mitochondrial dysfunction leading to neuronal cell death [8McCormack AL, Atienza JG, Johnston LC, Andersen JK, Vu S, Di Monte DA. Role of oxidative stress in paraquat-induced dopaminergic cell degeneration. J Neurochem 2005; 93(4): 1030-7.
[http://dx.doi.org/10.1111/j.1471-4159.2005.03088.x] [PMID: 15857406] , 9Henchcliffe C, Beal MF. Mitochondrial biology and oxidative stress in Parkinson disease pathogenesis. Nat Clin Pract Neurol 2008; 4(11): 600-9.
[http://dx.doi.org/10.1038/ncpneuro0924] [PMID: 18978800] ]. Neurotoxic compounds that target complex-I of mitochondria can induce dopaminergic cell death to produce PD condition. Defects in mitochondrial function in substantia nigra of the brain in PD patients have been observed which gives a compelling evidence for the role of mitochondrial dysfunction in the pathogenesis of PD [10Schapira AHV, Gegg M. Mitochondrial contribution to Parkinson’s disease pathogenesis. Parkinsons Dis 2011; 2011: 159160.
[http://dx.doi.org/10.4061/2011/159160] ].
Drosophila melanogaster is widely used as a model to study neurodegenerative diseases including PD to elucidate the mechanisms involved in the disease [11Feany MB, Bender WW. Drosophila model of Parkinson’s disease. Nature 2000; 404(6776): 394-8.
[http://dx.doi.org/10.1038/35006074] [PMID: 10746727] -13Lu B, Vogel H. Drosophila models of neurodegenerative diseases. Annu Rev Pathol 2009; 4: 315-42.
[http://dx.doi.org/10.1146/annurev.pathol.3.121806.151529] [PMID: 18842101] ]. Genetic and pesticide induced PD models of Drosophila are useful to screen natural bioactive compounds for their neuroprotective potential. The acute PQ model of PD in Drosophila is widely used to evaluate the neuroprotective potential of bioactive compounds [14Bonilla E, Medina-Leendertz S, Díaz S. Extension of life span and stress resistance of Drosophila melanogaster by long-term supplementation with melatonin. Exp Gerontol 2002; 37(5): 629-38.
[http://dx.doi.org/10.1016/S0531-5565(01)00229-7] [PMID: 11909680] -16Hosamani R. Prophylactic treatment with Bacopa monnieri leaf powder mitigates paraquat-induced oxidative perturbations and lethality in Drosophila melanogaster. Indian J Biochem Biophys 2010; 47(2): 75-82.
[PMID: 20521619] ]. However, the acute PQ model of Drosophila to induce PD is inadequate because of high mortality, reversible symptoms of motor impairment and neurodegeneration [17Navarro JA, Heßner S, Yenisetti SC, et al. Analysis of dopaminergic neuronal dysfunction in genetic and toxin-induced models of Parkinson’s disease in Drosophila. J Neurochem 2014; 131(3): 369-82.
[http://dx.doi.org/10.1111/jnc.12818] [PMID: 25040725] , 18Niveditha S, Ramesh SR, Shivanandappa T. Paraquat-induced movement disorder in relation to neurodegeneration in the brain of Drosophila melanogaster. Neurochem Res 2017; 42(11): 3310-20.
[http://dx.doi.org/10.1007/s11064-017-2373-y] [PMID: 28819888] ]. We have used a revised sub-acute model wherein flies exposed to multiple-dose (sub-lethal) of PQ replicates PD-like phenotype showing irreversible neurodegeneration in the brain and therefore, a more appropriate model to test the efficacy of neuroprotective agents [18Niveditha S, Ramesh SR, Shivanandappa T. Paraquat-induced movement disorder in relation to neurodegeneration in the brain of Drosophila melanogaster. Neurochem Res 2017; 42(11): 3310-20.
[http://dx.doi.org/10.1007/s11064-017-2373-y] [PMID: 28819888] ].
A large body of evidence suggests that phytochemicals possess health-promoting properties attributed to their ability to alleviate oxidative stress [15Park JH, Jung JW, Ahn YJ, Kwon HW. Neuroprotective properties of phytochemicals against paraquat-induced oxidative stress and neurotoxicity in Drosophila melanogaster. Pestic Biochem Physiol 2012; 104: 118-25.
[http://dx.doi.org/10.1016/j.pestbp.2012.07.006] , 19Kim SI, Jung JW, Ahn YJ, Restifo LL, Kwon HW. Drosophila as a model system for studying lifespan and neuroprotective activities of plant-derived compounds. J Asia Pac Entomol 2011; 14: 509-17.
[http://dx.doi.org/10.1016/j.aspen.2011.07.001] -21Pandey KB, Rizvi SI. Plant polyphenols as dietary antioxidants in human health and disease. Oxid Med Cell Longev 2009; 2(5): 270-8.
[http://dx.doi.org/10.4161/oxim.2.5.9498] [PMID: 20716914] ]. Since oxidative stress-induced mitochondrial dysfunction, is implicated in the pathogenesis of PD, treatment with antioxidants could be a useful strategy in ameliorating neurodegeneration, and slowing down the disease progression [22Yacoubian TA, Standaert DG. Targets for neuroprotection in Parkinson’s disease. Biochim Biophys Acta 2009; 1792: 676-87.].
Many bioactive compounds have been tested for their ability to protect against neurodegeneration, and those possessing antioxidant properties such as vitamins and flavonoids are of particular interest. Quercetin, a natural flavonoid antioxidant, showed neuroprotective activity in several neurodegenerative disease models including PD [23Lv C, Hong T, Yang Z, et al. Effect of Quercetin in the 1-Methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine-Induced mouse model of Parkinson’s Disease. Evid-Based Complement Alternat Med 2012; 1-6. 2012-25Sabogal-Guáqueta AM, Muñoz-Manco JI, Ramírez-Pineda JR, Lamprea-Rodriguez M, Osorio E, Cardona-Gómez GP. The flavonoid quercetin ameliorates Alzheimer’s disease pathology and protects cognitive and emotional function in aged triple transgenic Alzheimer’s disease model mice. Neuropharmacology 2015; 93: 134-45.
[http://dx.doi.org/10.1016/j.neuropharm.2015.01.027] [PMID: 25666032] ]. Nicotinamide, the amide form of niacin, and a precursor of the cofactor, nicotinamide adenine dinucleotide (NAD+), acts as an endogenous antioxidant [26Kirsch M, De Groot H. NAD(P)H, a directly operating antioxidant? FASEB J 2001; 15(9): 1569-74.
[http://dx.doi.org/10.1096/fj.00-0823hyp] [PMID: 11427489] ] and reported to possess neuroprotective activity in several PD models [27Anderson DW, Bradbury KA, Schneider JS. Neuroprotection in Parkinson models varies with toxin administration protocol. Eur J Neurosci 2006; 24(11): 3174-82.
[http://dx.doi.org/10.1111/j.1460-9568.2006.05192.x] [PMID: 17156378] , 28Jia H, Li X, Gao H, et al. High doses of nicotinamide prevent oxidative mitochondrial dysfunction in a cellular model and improve motor deficit in a Drosophila model of Parkinson’s disease. J Neurosci Res 2008; 86(9): 2083-90.
[http://dx.doi.org/10.1002/jnr.21650] [PMID: 18381761] ].
The edible roots of Decalepis hamiltonii (Dh) are a source of potent cocktail of natural antioxidants [29Srivastava A, Harish SR, Shivanandappa T. Antioxidant activity of the roots of Decalepis hamiltonii (Wight & Arn.). Food Sci Technol 2006; 39: 1059-65.] and, several novel antioxidant molecules have been isolated and characterized [30Harish R, Divakar S, Srivastava A, Shivanandappa T. Isolation of antioxidant compounds from the methanolic extract of the roots of Decalepis hamiltonii (Wight and Arn.). J Agric Food Chem 2005; 53(20): 7709-14.
[http://dx.doi.org/10.1021/jf051047c] [PMID: 16190621] , 31Srivastava A, Harish R, Shivanandappa T. Novel antioxidant compounds from the aqueous extract of the roots of Decalepis hamiltonii (Wight and Arn.) and their inhibitory effect on low-density lipoprotein oxidation. J Agric Food Chem 2006; 54(3): 790-5.
[http://dx.doi.org/10.1021/jf052433x] [PMID: 16448184] ]. The natural bioactive compounds from Dh have been shown to exhibit cytoprotective potential against oxidative stress-induced cell injury [32Srivastava A, Rao LJ, Shivanandappa T. Isolation of ellagic acid from the aqueous extract of the roots of Decalepis hamiltonii: Antioxidant activity and cytoprotective effect. Food Chem 2007; 103: 224-33.
[http://dx.doi.org/10.1016/j.foodchem.2006.08.010] -35Srivastava A, Rao LJ, Shivanandappa T. 14-aminotetradecanoic acid exhibits antioxidant activity and ameliorates xenobiotics-induced cytotoxicity. Mol Cell Biochem 2012; 364(1-2): 1-9.
[http://dx.doi.org/10.1007/s11010-011-1196-4] [PMID: 22198290] ] Furthermore, hepatoprotective [36Srivastava A, Shivanandappa T. Hepatoprotective effect of the aqueous extract of the roots of Decalepis hamiltonii against ethanol-induced oxidative stress in rats. Hepatol Res 2006; 35(4): 267-75.
[http://dx.doi.org/10.1016/j.hepres.2006.04.011] [PMID: 16777477] ] and neuroprotective [37Srivastava A, Shivanandappa T. Decalepis hamiltonii roots boost antioxidant status of rat liver and brain. J Sci Food Agric 2009; 89: 2461-6.
[http://dx.doi.org/10.1002/jsfa.3748] -39Srivastava A, Shivanandappa T. Differential cholinesterase inhibition in the rat brain regions by dichlorvos and protective effect of Decalepis hamiltonii roots. Neurotoxicology 2011; 32(6): 931-4.
[http://dx.doi.org/10.1016/j.neuro.2011.04.007] [PMID: 21571001] ] activity of the roots of Dh against hepatotoxic and neurotoxic chemicals in vivo has been reported. Ellagic acid, a polyphenolic antioxidant from Dh, exhibits in vitro free radical scavenging activity as well as cytoprotective activity against xenobiotic-induced oxidative stress in primary hepatocytes and Ehrlich Ascites tumor cells [32Srivastava A, Rao LJ, Shivanandappa T. Isolation of ellagic acid from the aqueous extract of the roots of Decalepis hamiltonii: Antioxidant activity and cytoprotective effect. Food Chem 2007; 103: 224-33.
[http://dx.doi.org/10.1016/j.foodchem.2006.08.010] ]. Recent reports suggest that ellagic acid mitigates neurodegeneration possibly via free radical scavenging activity [40Dolatshahi M, Farbood Y, Sarkaki A, Mansouri SM, Khodadadi A. Ellagic acid improves hyperalgesia and cognitive deficiency in 6-hydroxidopamine induced rat model of Parkinson’s disease. Iran J Basic Med Sci 2015; 18(1): 38-46.
[PMID: 25810874] -42Ahmed T, Setzer WN, Nabavi SF, et al. Insights into effects of ellagic acid on the nervous system: A mini review. Curr Pharm Des 2016; 22(10): 1350-60.
[http://dx.doi.org/10.2174/1381612822666160125114503] [PMID: 26806345] ]. 4Hydroxyisophthalic acid (4- HIPA or DHA-I), a novel bioactive molecule isolated from the aqueous extract of Dh roots, shows potent in vitro free radical scavenging activity and cytoprotective activity against xenobiotic-induced oxidative stress in primary hepatocytes and Ehrlich Ascites tumor cells [34Srivastava A, Rao LJ, Shivanandappa T. A novel cytoprotective antioxidant: Hydroxyisophthalic acid. Food Chem 2012; 132: 195965.
[http://dx.doi.org/10.1016/j.foodchem.2011.12.032] ]. Furthermore, we have shown that DHA-I exhibits neuroprotective potential by attenuating tau neuropathy in transgenic Drosophila model [43Haddadi M, Jahromi SR, Nongthomba U, Shivanandappa T, Ramesh SR. 4-Hydroxyisophthalic acid from Decalepis hamiltonii rescues the neurobehavioral deficit in transgenic Drosophila model of taupathies. Neurochem Int 2016; 100: 78-90.
[http://dx.doi.org/10.1016/j.neuint.2016.09.007] [PMID: 27615061] ]. The objective of this study was to compare the antioxidant and neuroprotective activity of DHA-I with the three natural antioxidants, ellagic acid, quercetin and nicotinamide, in the Drosophila model of PQ neurotoxicity and motor impairment.
2. MATERIALS AND METHODS
2.1. Chemicals and Antioxidant Compounds
Paraquat dichloride (PQ), 2’7’- dichlorofluorescin diacetate (DCFH-DA), acetylthiocholine iodide (ATCI), pyrogallol, bovine serum albumin, thiobarbituric acid (TBA), sodium lauryl sulphate (SDS) were purchased from Sigma Chemical Co. (St. Louis, MO, USA).
Nicotinamide (NA), ellagic acid (EA) and quercetin (Que) were purchased from Sigma Chemical Co. (St. Louis, MO, USA).
5, 5’-dithio-bis-(2-nitrobenzoic acid) (DTNB), hydrogen peroxide (H2O2), trichloroacetic acid (TCA) and other chemicals were purchased from Sisco Research Laboratories, Mumbai, India.
The isolation of DHA-I from the aqueous extract of Dh roots was done by silica gel column chromatography and RP-HPLC, and characterized by UV, IR, LC-MS, and NMR spectroscopic techniques as described earlier [31Srivastava A, Harish R, Shivanandappa T. Novel antioxidant compounds from the aqueous extract of the roots of Decalepis hamiltonii (Wight and Arn.) and their inhibitory effect on low-density lipoprotein oxidation. J Agric Food Chem 2006; 54(3): 790-5.
[http://dx.doi.org/10.1021/jf052433x] [PMID: 16448184] , 34Srivastava A, Rao LJ, Shivanandappa T. A novel cytoprotective antioxidant: Hydroxyisophthalic acid. Food Chem 2012; 132: 195965.
[http://dx.doi.org/10.1016/j.foodchem.2011.12.032] ].
2.2. Drosophila Culture
Wild-type D. melanogaster (Oregon K) adult flies were obtained from Drosophila Stock centre, Department of Zoology, Manasagangotri, University of Mysore, Karnataka, India. All flies were maintained in a 150 ml culture bottle containing 30 ml of standard wheat cream–agar medium seeded with yeast. Flies were reared at 22±1°C and 70–80% relative humidity for all the experiments.
2.3. Treatment
The experimental diets containing DHA-I (dissolved in phosphate buffered saline), nicotinamide (dissolved in distilled water), ellagic acid (dissolved in 0.6% DMSO) and quercetin (dissolved in distilled water) were prepared at a final concentration of 0.01%, 0.02% and 0.05% (w/v) in wheat cream-agar diet. Newly eclosed flies fed with control diet (without antioxidants) and experimental diets for five days were transferred to the vials containing filter paper soaked with 5% sucrose solution and acute dose of PQ (20 mM). Exposure to the acute dose of PQ is associated with high mortality in adult flies and the number of dead flies was scored after 24h of exposure. Based on survival against acute PQ toxicity, effective doses of each of the antioxidant compounds were determined. For each tested group, six replicates of ten flies each per sex were tested and expressed as percentage survival.
Flies were exposed to multiple sub-lethal dose of PQ (5.375mM - one-fourth of LC50) to induce oxidative stress and PD-like symptoms. Briefly, six-day-old flies were transferred to the vials containing filter paper soaked with 5% sucrose solution and 5.375 mM PQ for 24h on every alternate day for 8 days [18Niveditha S, Ramesh SR, Shivanandappa T. Paraquat-induced movement disorder in relation to neurodegeneration in the brain of Drosophila melanogaster. Neurochem Res 2017; 42(11): 3310-20.
[http://dx.doi.org/10.1007/s11064-017-2373-y] [PMID: 28819888] ]. Flies were transferred back to normal wheat-cream agar medium after every PQ exposure to minimize any profound effects of PQ as well as sucrose diet on the survivability of flies [44Rzezniczak TZ, Douglas LA, Watterson JH, Merritt TJ. Paraquat administration in Drosophila for use in metabolic studies of oxidative stress. Anal Biochem 2011; 419(2): 345-7.
[http://dx.doi.org/10.1016/j.ab.2011.08.023] [PMID: 21910964] ].
For neuroprotection studies, newly eclosed flies were fed with control diet or experimental diets containing following compounds: DHA-I, nicotinamide, ellagic acid and quercetin at a final concentration of 0.02% (effective dose) for next five days. Following feeding process, flies were exposed to 5.375 mM of PQ for 24h on alternative day for 8 days and transferred to the vials containing respective diets after each PQ exposure.
2.4. Climbing Activity (Negative Geotaxis Assay)
To determine the effect of PQ on locomotion pattern, the climbing ability of flies was monitored by negative geotaxis assay. Briefly, ten flies were introduced into a vertical glass column (standard length, 25 cm). After a brief recovery period, flies were gently tapped to the bottom and then allowed to climb [11Feany MB, Bender WW. Drosophila model of Parkinson’s disease. Nature 2000; 404(6776): 394-8.
[http://dx.doi.org/10.1038/35006074] [PMID: 10746727] ]. Effect of antioxidant compounds on PQ-induced locomotor impairment was monitored from the climbing response. The number of flies climbed beyond the minimum distance of 12 cm in 20s of interval was recorded. For each tested group, six replicates of ten flies each per sex were tested and expressed as an average of six replicates.
2.5. Biochemical Assays
Both control and treated flies were subjected to cold anesthesia for 10min. 50 fly heads were homogenized in 500 µl of respective assay buffers and centrifuged at 2500×g for 10min at 4°C. The supernatant was used to determine the levels of ROS, lipid peroxidation (LPO), reduced glutathione (GSH), the activity of antioxidant enzymes (SOD and catalase) and acetylcholinesterase (AChE).
2.5.1. Reactive Oxygen Species
ROS was quantified by fluorimetric method as described previously [45LeBel CP, Ischiropoulos H, Bondy SC. Evaluation of the probe 2′,7′-dichlorofluorescin as an indicator of reactive oxygen species formation and oxidative stress. Chem Res Toxicol 1992; 5(2): 227-31.
[http://dx.doi.org/10.1021/tx00026a012] [PMID: 1322737] ]. The reaction involves ROS-mediated conversion of DCFH-DA into a fluorescent product, 2’7’- dichlorofluorescin (DCF) which was measured using multimode plate reader with an excitation wavelength of 488 nm and emission at 525 nm. ROS was quantified using DCF standard curve and expressed as µmoles of DCF formed/min/mg protein. For each tested group, three replicates of 50 flies each per sex were used.
2.5.2. Lipid Peroxidation
LPO was assayed by measuring the thiobarbituric acid reactive substances. Briefly, 500 µl of tissue homogenate was heated with the reaction mixture containing 1.5 ml of 20% (v/v) acetic acid (pH 3.5), 1.5 ml of 0.8% (w/v) TBA, and 200 µl of 8% (w/v) SDS in boiling water bath for 1h. After cooling, adducts were extracted in 3 ml of 1-butanol. Following the centrifugation, the absorbance of the supernatant was determined at 532 nm and expressed as malondialdehyde equivalents [46Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 1979; 95(2): 351-8.
[http://dx.doi.org/10.1016/0003-2697(79)90738-3] [PMID: 36810] ]. For each tested group, three replicates of 50 flies each per sex were used.
2.5.3. Reduced Glutathione
Fly head-homogenate was prepared in 1ml of 5% (w/v) TCA, centrifuged at 2,500×g for 10min at 4°C. The deproteinized supernatant was subjected to GSH estimation by using Ellman’s reagent. Briefly, 200 µl of tissue homogenate was incubated with 2.8 ml of 0.2M tris-HCl buffer (pH 8) and 50 µl of 10 mM DTNB at room temperature for 5 min. The yellow colored product was measured at 412 nm. GSH content was quantified using GSH standard curve and expressed as µg of GSH/mg protein [47Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys 1959; 82(1): 70-7.
[http://dx.doi.org/10.1016/0003-9861(59)90090-6] [PMID: 13650640] ]. For each tested group, three replicates of 50 flies each per sex were used.
2.5.4. Antioxidant Enzymes
SOD activity was determined by measuring the inhibition of pyrogallol autoxidation. Briefly, the reaction was started by adding 0.5 ml of 2 mM pyrogallol to the reaction mixture containing 500 µl of tissue homogenate, 0.5 ml of distilled water and 2 ml of 0.1 M tris-HCl buffer (pH 8.2). The change in absorbance was monitored for 3 min using spectrophotometer at 420 nm. The activity was expressed as enzyme units required to inhibit 50% pyrogallol autooxidation. Cyanide selectively inhibits the activity of Cu, Zn-SOD and allows the measurement of Mn-SOD activity. By using Potassium Cyanide (1mM), activities of both Cu, Zn- SOD and Mn-SOD were determined [48Marklund S, Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem 1974; 47(3): 469-74.
[http://dx.doi.org/10.1111/j.1432-1033.1974.tb03714.x] [PMID: 4215654] ]. For each tested group, three replicates of 50 flies each per sex were used.
Catalase activity was determined by measuring the rate of H2O2 decomposition as described previously [49Aebi H. Catalase.Methods of Enzymatic Analysis 3rd ed. 1983; 273-86.]. Briefly, 50 µl of 1% (v/v) H2O2 was added to 1 ml reaction mixture containing 50 µl homogenate and 950 µl of 0.05M phosphate buffer (pH 7). The change in absorbance was monitored for 3 min using spectrophotometer at 240 nm and expressed as µmoles of H2O2 decomposed/min/mg protein.
For each tested group, three replicates of 50 flies each per sex were used.
2.5.5. Acetylcholinesterase
AChE activity was assayed by measuring the rate of hydrolysis of ATCI in 0.1 M phosphate buffer (pH 8). The reaction was started by adding 200 µl of tissue homogenate to 2.8 ml reaction mixture containing 250 µl 10 mM DTNB, 100 µl 30 mM ATCI and 2.45 ml of phosphate buffer. The change in absorbance was monitored for 3mins using spectrophotometer at 412nm and expressed as µmoles of ATCI hydrolyzed/min/mg protein [50Ellman GL, Courtney KD, Andres V Jr, Feather-Stone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 1961; 7: 88-95.
[http://dx.doi.org/10.1016/0006-2952(61)90145-9] [PMID: 13726518] ]. For each tested group, three replicates of 50 flies each per sex were used.
2.6. Statistical Analysis
All data were analyzed by one-way analysis of variance (ANOVA) followed by post hoc ‘Tukey’ test using SPSS software. Data were expressed as mean±SE and the significant difference (p<0.05) was determined.
3. RESULTS
3.1. Paraquat-induced Mortality
In flies exposed to the acute dose of PQ (20 mM), a significant mortality was observed after 24h. Treatment with different antioxidant compounds improved the survival of adult flies against PQ toxicity than control flies (Fig. 1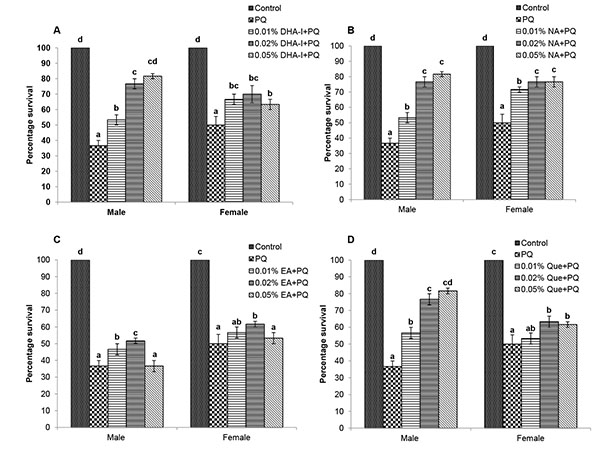 ). Both male and female flies fed with different antioxidant compounds exhibited dose-dependent survival against PQ- toxicity. The percentage survival of males fed with 0.02% and 0.05% of DHA-I, nicotinamide or quercetin was significantly higher compared to other doses whereas ellagic acid was effective against PQ toxicity only at 0.02%. Also, the survival of females fed with 0.02% of DHA-I or ellagic acid, 0.02% and 0.05% of nicotinamide or quercetin was significantly higher compared to other doses of respective compounds. Based on the effective doses against acute PQ-toxicity, 0.02% was used as an optimal concentration to compare the neuroprotective action of these compounds against PQ-induced oxidative stress and neural dysfunctions.
). Both male and female flies fed with different antioxidant compounds exhibited dose-dependent survival against PQ- toxicity. The percentage survival of males fed with 0.02% and 0.05% of DHA-I, nicotinamide or quercetin was significantly higher compared to other doses whereas ellagic acid was effective against PQ toxicity only at 0.02%. Also, the survival of females fed with 0.02% of DHA-I or ellagic acid, 0.02% and 0.05% of nicotinamide or quercetin was significantly higher compared to other doses of respective compounds. Based on the effective doses against acute PQ-toxicity, 0.02% was used as an optimal concentration to compare the neuroprotective action of these compounds against PQ-induced oxidative stress and neural dysfunctions.
After 24h of PQ exposure, flies fed with 0.02% of DHA-I, quercetin and nicotinamide showed better survival than the fly group fed with ellagic acid. However, there were sex differences in the protective action of antioxidants against PQ toxicity. DHA-I and quercetin showed protective action against PQ-induced mortality in both the sexes; whereas, in females, the protective effect of nicotinamide on survival was higher compared to DHA-I and quercetin (Fig. 2 ).
).
3.2. Locomotor Deficits
Flies exposed to multiple-dose of PQ resulted in severe locomotor impairment as evident from the negative geotaxis assay. Flies tend to stay at the bottom of the column which indicates deleterious effects of PQ on the climbing ability, characteristic of PD. Dietary supplementation of the antioxidant compounds was able to alleviate the locomotor deficits caused by PQ exposure (Fig. 3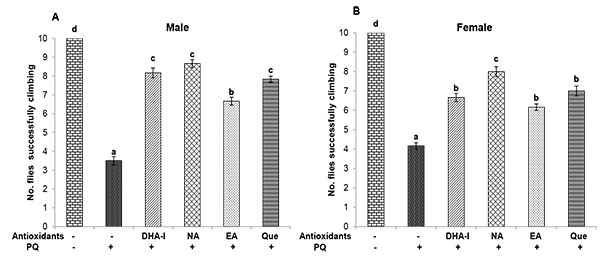 ). Protective action of DHA-I, quercetin and nicotinamide on climbing ability was markedly higher compared to ellagic acid in both the sexes. However, in females, nicotinamide provided better protection against PQ-induced locomotor impairment compared to quercetin and DHA-I.
). Protective action of DHA-I, quercetin and nicotinamide on climbing ability was markedly higher compared to ellagic acid in both the sexes. However, in females, nicotinamide provided better protection against PQ-induced locomotor impairment compared to quercetin and DHA-I.
3.3. Oxidative Stress Markers
Flies exposed to multiple dose of PQ showed a significant increase in ROS levels in both sexes (Figs. 4A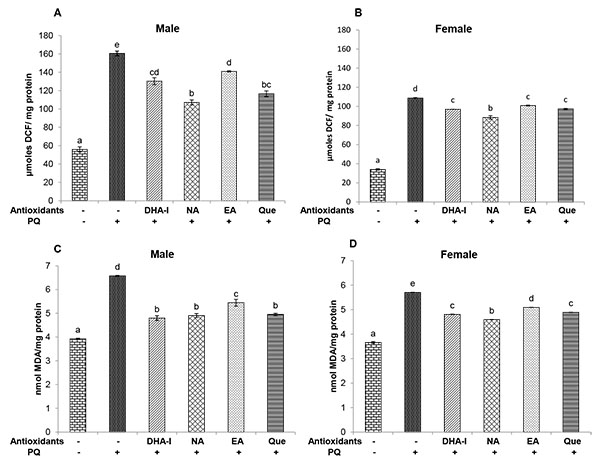 and 4B
and 4B ). There was also a marked increase in the level of LPO in PQ-treated flies (Figs. 4C
). There was also a marked increase in the level of LPO in PQ-treated flies (Figs. 4C and 4D
and 4D ). All four fly groups fed with the antioxidant compounds showed decreased levels of ROS and LPO when compared to the PQ-only treated flies. Among the antioxidant compounds, nicotinamide caused a greater reduction in ROS levels followed by quercetin, DHA-I, and ellagic acid. Also, administration of DHA-I, nicotinamide and quercetin caused a significant decrease in LPO level compared to ellagic acid in both the sexes.
). All four fly groups fed with the antioxidant compounds showed decreased levels of ROS and LPO when compared to the PQ-only treated flies. Among the antioxidant compounds, nicotinamide caused a greater reduction in ROS levels followed by quercetin, DHA-I, and ellagic acid. Also, administration of DHA-I, nicotinamide and quercetin caused a significant decrease in LPO level compared to ellagic acid in both the sexes.
Exposure to PQ resulted in decreased level of GSH compared to control. Treatment with DHA-I, nicotinamide and quercetin showed an increase in GSH content; whereas, ellagic acid did not exert any influence on GSH level (Fig. 5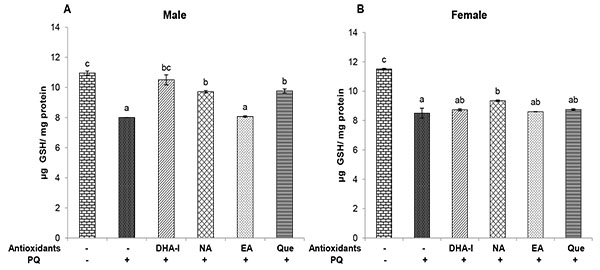 ). Interestingly, in females, the ameliorative effect of nicotinamide against PQ-induced GSH depletion was higher compared to DHA-I and quercetin.
). Interestingly, in females, the ameliorative effect of nicotinamide against PQ-induced GSH depletion was higher compared to DHA-I and quercetin.
DHA-I treatment prevented the PQ-induced changes in SOD activity in both sexes. The protective effect of DHA-I on Mn-SOD was clearly higher than that of Cu-Zn SOD. Similar protective effect of quercetin on PQ-induced SOD activity was seen. Interestingly, there were sex differences in the efficacy of the antioxidants. DHA-I and quercetin showed protective action on SOD activity in both the sexes; however, in females, the protective effect of nicotinamide against PQ-induced changes in SOD activity was higher compared to DHA-I and quercetin (Fig. 6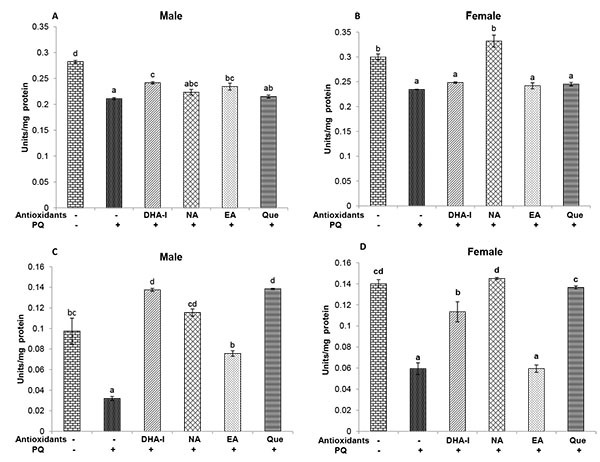 ). Catalase, on the other hand, was significantly increased in flies exposed to PQ and administration of antioxidant compounds prevented these alterations to varying degree. DHA-I and quercetin were more effective in prevention of changes in catalase activity than nicotinamide and ellagic acid (Fig. 7
). Catalase, on the other hand, was significantly increased in flies exposed to PQ and administration of antioxidant compounds prevented these alterations to varying degree. DHA-I and quercetin were more effective in prevention of changes in catalase activity than nicotinamide and ellagic acid (Fig. 7 ).
).
3.4. Acetylcholinesterase
Flies exhibited a significant increase in the activity of AChE when exposed to PQ and dietary supplementation of antioxidant compounds prevented these alterations. Among the antioxidants, DHA-I, quercetin and nicotinamide were more effective in the prevention of changes in AChE activity than ellagic acid (Fig. 8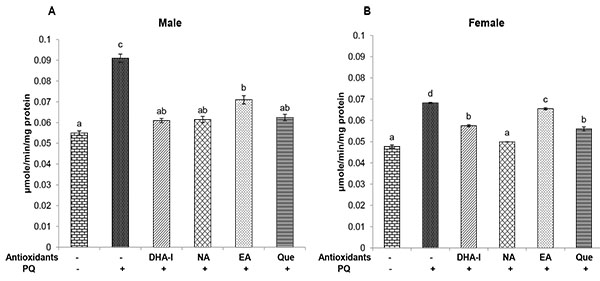 ).
).
Flies treated with the natural antioxidants alone did not show significant differences in toxicity and biochemical markers compared to that of controls (Data not shown).
4. DISCUSSION
The ability of antioxidants to quench the oxidative stress-mediated damage is believed to contribute to their therapeutic potential in preventing or slowing down neurodegeneration. Several studies have demonstrated that the antioxidant compounds protect neuronal cells by neutralizing the excessive free radicals and/or by enhancing the antioxidant defenses [52Ishige K, Schubert D, Sagara Y. Flavonoids protect neuronal cells from oxidative stress by three distinct mechanisms. Free Radic Biol Med 2001; 30(4): 433-46.
[http://dx.doi.org/10.1016/S0891-5849(00)00498-6] [PMID: 11182299] , 53Kanski J, Aksenova M, Stoyanova A, Butterfield DA. Ferulic acid antioxidant protection against hydroxyl and peroxyl radical oxidation in synaptosomal and neuronal cell culture systems in vitro: Structure-activity studies. J Nutr Biochem 2002; 13(5): 273-81.
[http://dx.doi.org/10.1016/S0955-2863(01)00215-7] [PMID: 12015157] ]. The involvement of oxidative stress in the initiation or progression of neurodegeneration provides the basis for considering antioxidant therapy as a prophylactic treatment for neurodegenerative diseases including PD.
Previous studies from our laboratory have shown that the antioxidant-rich root extract of Dh exhibits neuroprotective action against acute PQ neurotoxicity and enhance the cognitive ability in Drosophila [54Haddadi M, Jahromi SR, Shivanandappa T, Ramesh SR. Decalepis hamiltonii root extract attenuates the age-related decline in the cognitive function in Drosophila melanogaster. Behav Brain Res 2013; 249: 8-14.
[http://dx.doi.org/10.1016/j.bbr.2013.04.017] [PMID: 23608486] , 55Jahromi SR, Haddadi M, Shivanandappa T, Ramesh SR. Neuroprotective effect of Decalepis hamiltonii in paraquat-induced neurotoxicity in Drosophila melanogaster: Biochemical and behavioral evidences. Neurochem Res 2013; 38(12): 2616-24.
[http://dx.doi.org/10.1007/s11064-013-1179-9] [PMID: 24173775] ]. The root extract of Dh also showed neuroprotective effects against PQ toxicity in αsynuclein transgenic flies and delayed the onset of PD-like symptoms, which could be attributed to its antioxidant constituents [56Jahromi SR, Haddadi M, Shivanandappa T, Ramesh SR. Attenuation of neuromotor deficits by natural antioxidants of Decalepis hamiltonii in transgenic Drosophila model of Parkinson’s disease. Neuroscience 2015; 293: 136-50.
[http://dx.doi.org/10.1016/j.neuroscience.2015.02.048] [PMID: 25754960] ]. DHA-I, the bioactive compound from Dh, showed neuroprotective activity against taupathy in Drosophila [43Haddadi M, Jahromi SR, Nongthomba U, Shivanandappa T, Ramesh SR. 4-Hydroxyisophthalic acid from Decalepis hamiltonii rescues the neurobehavioral deficit in transgenic Drosophila model of taupathies. Neurochem Int 2016; 100: 78-90.
[http://dx.doi.org/10.1016/j.neuint.2016.09.007] [PMID: 27615061] ]. In the present study, the neuroprotective efficacy of DHA-I was compared with the three natural antioxidants against multiple-dose PQ neurotoxicity that induces locomotor impairment and oxidative stress. We have earlier shown that multiple-dose of PQ treatment causes oxidative stress-induced neurodegeneration and movement disorder typical of PD [18Niveditha S, Ramesh SR, Shivanandappa T. Paraquat-induced movement disorder in relation to neurodegeneration in the brain of Drosophila melanogaster. Neurochem Res 2017; 42(11): 3310-20.
[http://dx.doi.org/10.1007/s11064-017-2373-y] [PMID: 28819888] ] suggesting the association between oxidative stress and PQ neurotoxicity. Our results show that the natural antioxidants suppressed PQ-induced oxidative stress as evident from decreased ROS and LPO with augmented GSH level, and improved survival and climbing ability. PQ treated flies showed overall decrease in the activity of total SOD. Interestingly, the activity of mitochondrial Mn-SOD, an enzyme involved in the detoxification of mitochondrial ROS, was markedly decreased, which could be associated with mitochondrial damage and neurodegeneration as reported in our previous study [18Niveditha S, Ramesh SR, Shivanandappa T. Paraquat-induced movement disorder in relation to neurodegeneration in the brain of Drosophila melanogaster. Neurochem Res 2017; 42(11): 3310-20.
[http://dx.doi.org/10.1007/s11064-017-2373-y] [PMID: 28819888] ]. Catalase activity, on the other hand, was increased upon PQ exposure. The activity of AChE, a general biochemical marker of neural function, is also elevated in PQ-treated flies. Administration of the antioxidant compounds showed varying effects on SOD, catalase and AChE activities in the fly head. Flies fed with the antioxidant compounds prevented the PQ-induced changes in the activity of Mn-SOD, indicating their ameliorative potential against oxidative stress-mediated mitochondrial damage involved in neurodegeneration. Also, antioxidant compounds attenuated the PQ-induced changes in the activity of catalase and AChE, suggesting the ameliorative effect of natural antioxidants in improving the antioxidant defenses and AChE activity involved in the cholinergic function.
Overall, the antioxidant compounds showed protective effect against PQ-induced oxidative stress and neurotoxicity in Drosophila as evident from better survivability, improved locomotor activity and enhanced antioxidant defenses. Our results are consistent with the previous studies which have reported that quercetin, ellagic acid and nicotinamide show neuroprotective activity in several models [27Anderson DW, Bradbury KA, Schneider JS. Neuroprotection in Parkinson models varies with toxin administration protocol. Eur J Neurosci 2006; 24(11): 3174-82.
[http://dx.doi.org/10.1111/j.1460-9568.2006.05192.x] [PMID: 17156378] , 28Jia H, Li X, Gao H, et al. High doses of nicotinamide prevent oxidative mitochondrial dysfunction in a cellular model and improve motor deficit in a Drosophila model of Parkinson’s disease. J Neurosci Res 2008; 86(9): 2083-90.
[http://dx.doi.org/10.1002/jnr.21650] [PMID: 18381761] , 40Dolatshahi M, Farbood Y, Sarkaki A, Mansouri SM, Khodadadi A. Ellagic acid improves hyperalgesia and cognitive deficiency in 6-hydroxidopamine induced rat model of Parkinson’s disease. Iran J Basic Med Sci 2015; 18(1): 38-46.
[PMID: 25810874] -42Ahmed T, Setzer WN, Nabavi SF, et al. Insights into effects of ellagic acid on the nervous system: A mini review. Curr Pharm Des 2016; 22(10): 1350-60.
[http://dx.doi.org/10.2174/1381612822666160125114503] [PMID: 26806345] , 57Abdalla FH, Schmatz R, Cardoso AM, et al. Quercetin protects the impairment of memory and anxiogenic-like behavior in rats exposed to cadmium: Possible involvement of the acetylcholinesterase and Na(+),K(+)-ATPase activities. Physiol Behav 2014; 135: 152-67.
[http://dx.doi.org/10.1016/j.physbeh.2014.06.008] [PMID: 24952260] ]. Nicotinamide, a precursor of NAD is vital to cellular oxidation-reduction reactions and acts as a cofactor for mitochondrial enzymes involved in ATP production [58Maiese K, Chong ZZ. Nicotinamide: necessary nutrient emerges as a novel cytoprotectant for the brain. Trends Pharmacol Sci 2003; 24(5): 228-32.
[http://dx.doi.org/10.1016/S0165-6147(03)00078-6] [PMID: 12767721] ]. Nicotinamide is known to inhibit the activity of poly ADP-ribose polymerase-1 (PARP-1), an enzyme activated by DNA damage that catalyzes the cleavage of NAD+ leading to ATP depletion and cell death [59Mandir AS, Przedborski S, Jackson-Lewis V, et al. Poly(ADP-ribose) polymerase activation mediates 1-methyl-4-phenyl-1, 2,3,6-tetrahydropyridine (MPTP)-induced parkinsonism. Proc Natl Acad Sci USA 1999; 96(10): 5774-9.
[http://dx.doi.org/10.1073/pnas.96.10.5774] [PMID: 10318960] , 60Anderson DW, Bradbury KA, Schneider JS. Broad neuroprotective profile of nicotinamide in different mouse models of MPTP-induced parkinsonism. Eur J Neurosci 2008; 28(3): 610-7.
[http://dx.doi.org/10.1111/j.1460-9568.2008.06356.x] [PMID: 18702732] ]. Furthermore, depletion of NAD+ leads to decreased activity of silent information regulator 2 (Sir2) family (sirtuins) involved in the regulation of aging/longevity. Sirtuins are NAD-dependent protein deacylases, which acts on a variety of proteins including transcription factors involved in the activation of antioxidant genes [61Santos L, Escande C, Denicola A. Potential modulation of sirtuins by oxidative stress. Oxid Med Cell Longev 2016; 2016: 9831825.
[http://dx.doi.org/10.1155/2016/9831825] ]. Interestingly, supplementation of nicotinamide is associated with exogenous replenishment of NAD+, which regulates sirtuin activity in the nucleus and mitochondria [62Imai S, Guarente L. It takes two to tango: NAD(+) and sirtuins in aging/longevity control. NPJ Aging Mech Dis 2016; 2: 16017.
[http://dx.doi.org/10.1038/npjamd.2016.17] [PMID: 28721271] ]. Nicotinamide decreases oxidative stress and improves mitochondrial functions as well as motor deficits in Drosophila model of PD [28Jia H, Li X, Gao H, et al. High doses of nicotinamide prevent oxidative mitochondrial dysfunction in a cellular model and improve motor deficit in a Drosophila model of Parkinson’s disease. J Neurosci Res 2008; 86(9): 2083-90.
[http://dx.doi.org/10.1002/jnr.21650] [PMID: 18381761] ]. Nicotinamide has also been shown to attenuate dopaminergic neurodegeneration in a mouse model of PD, possibly by PARP-1 inhibition, increased NAD+ levels or both [60Anderson DW, Bradbury KA, Schneider JS. Broad neuroprotective profile of nicotinamide in different mouse models of MPTP-induced parkinsonism. Eur J Neurosci 2008; 28(3): 610-7.
[http://dx.doi.org/10.1111/j.1460-9568.2008.06356.x] [PMID: 18702732] ]. Quercetin, on the other hand, exhibits diverse pharmacological effects including anti-inflammatory and antioxidant activity with the ability to enhance antioxidant defenses [63Costa LG, Garrick JM, Roquè PJ, Pellacani C, Pellacani C. Mechanisms of neuroprotection by quercetin: counteracting oxidative stress and more. Oxid Med Cell Longev 2016; 2016: 1-10.
[http://dx.doi.org/10.1155/2016/2986796] ]. The ability of quercetin to modulate GSH redox system has been attributed to its protective action against oxidative stress-induced neuronal cell death in cell cultures [64Arredondo F, Echeverry C, Abin-Carriquiry JA, et al. After cellular internalization, quercetin causes Nrf2 nuclear translocation, increases glutathione levels, and prevents neuronal death against an oxidative insult. Free Radic Biol Med 2010; 49(5): 738-47.
[http://dx.doi.org/10.1016/j.freeradbiomed.2010.05.020] [PMID: 20554019] ]. Quercetin rescues mitochondrial dysfunction possibly through the activation of AMPactivated protein kinase (AMPK) pathway [65Wang DM, Li SQ, Wu WL, Zhu XY, Wang Y, Yuan H-Y. Effects of long-term treatment with quercetin on cognition and mitochondrial function in a mouse model of Alzheimer’s disease. Neurochem Res 2014; 39(8): 1533-43.
[http://dx.doi.org/10.1007/s11064-014-1343-x] [PMID: 24893798] ]. Similarly, ellagic acid has been reported to inhibit neuroinflammation and associated cell death in the brain, which could be related to its anti-inflammatory as well as antioxidant activity [41Farbood Y, Sarkaki A, Dolatshahi M, Taqhi Mansouri SM, Khodadadi A. Ellagic acid protects the brain against 6-Hydroxydopamine induced neuroinflammation in a rat model of Parkinson’s Disease. Basic Clin Neurosci 2015; 6(2): 83-9.
[PMID: 27307952] ]. Ellagic acid mitigates mitochondrial dysfunction by suppressing mitochondrial ROS production and loss of membrane potential [42Ahmed T, Setzer WN, Nabavi SF, et al. Insights into effects of ellagic acid on the nervous system: A mini review. Curr Pharm Des 2016; 22(10): 1350-60.
[http://dx.doi.org/10.2174/1381612822666160125114503] [PMID: 26806345] ]. Mitochondrial dysfunction due to increased production of ROS is believed to contribute to neurodegenerative events in PD, and maintenance of the integrity of mitochondria is considered to be a promising therapeutic strategy in PD [66Moreira PI, Zhu X, Wang X, et al. Mitochondria: A therapeutic target in neurodegeneration. Biochim Biophys Acta - Mol Basis Dis 2010; 1802: 212-0., 67Bose A, Beal MF. Mitochondrial dysfunction in Parkinson’s disease. J Neurochem 2016; 139(Suppl. 1): 216-31.
[http://dx.doi.org/10.1111/jnc.13731] [PMID: 27546335] ]. The natural antioxidants employed in our study showed varying degrees of protection against PQ neurotoxicity and, also exhibited sex differences in their action. The protective effect of DHA-I was comparable to that of nicotinamide as well as quercetin and was better than ellagic acid against PQinduced oxidative stress. Our results show that neuroprotective action of DHA-I and the natural antioxidants may act via attenuation of mitochondrial oxidative stress which is implicated in neuronal dysfunction.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
HUMAN AND ANIMAL RIGHTS
No Animals/Humans were used for studies that are base of this research.
CONSENT FOR PUBLICATION
Not applicable.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
The first author thank the Department of Science and Technology, Government of India, for the financial support under INSPIRE fellowship program. Thanks are also due to the Chairperson, Department of Zoology, University of Mysore, Mysuru, for the facilities.
REFERENCES
| [1] | Halliwell B. Free radicals, antioxidants, and human disease: Curiosity, cause, or consequence? Lancet 1994; 344(8924): 721-4. [http://dx.doi.org/10.1016/S0140-6736(94)92211-X] [PMID: 7915779] |
| [2] | Chen X, Guo C, Kong J. Oxidative stress in neurodegenerative diseases. Neural Regen Res 2012; 7(5): 376-85. [PMID: 25774178] |
| [3] | McCormack AL, Thiruchelvam M, Manning-Bog AB, et al. Environmental risk factors and Parkinson’s disease: Selective degeneration of nigral dopaminergic neurons caused by the herbicide paraquat. Neurobiol Dis 2002; 10(2): 119-27. [http://dx.doi.org/10.1006/nbdi.2002.0507] [PMID: 12127150] |
| [4] | Ossowska K, Wardas J, Smiałowska M, et al. A slowly developing dysfunction of dopaminergic nigrostriatal neurons induced by long-term paraquat administration in rats: An animal model of preclinical stages of Parkinson’s disease? Eur J Neurosci 2005; 22(6): 1294-304. [http://dx.doi.org/10.1111/j.1460-9568.2005.04301.x] [PMID: 16190885] |
| [5] | Chaudhuri A, Bowling K, Funderburk C, et al. Interaction of genetic and environmental factors in a Drosophila parkinsonism model. J Neurosci 2007; 27(10): 2457-67. [http://dx.doi.org/10.1523/JNEUROSCI.4239-06.2007] [PMID: 17344383] |
| [6] | Zhang XF, Thompson M, Xu YH. Multifactorial theory applied to the neurotoxicity of paraquat and paraquat-induced mechanisms of developing Parkinson’s disease. Lab Invest 2016; 96(5): 496-507. [http://dx.doi.org/10.1038/labinvest.2015.161] [PMID: 26829122] |
| [7] | Fridovich I, Hassan HM. Paraquat and the exacerbation of oxygen toxicity. Trends Biochem Sci 1979; 4: 113-5. [http://dx.doi.org/10.1016/0968-0004(79)90395-5] |
| [8] | McCormack AL, Atienza JG, Johnston LC, Andersen JK, Vu S, Di Monte DA. Role of oxidative stress in paraquat-induced dopaminergic cell degeneration. J Neurochem 2005; 93(4): 1030-7. [http://dx.doi.org/10.1111/j.1471-4159.2005.03088.x] [PMID: 15857406] |
| [9] | Henchcliffe C, Beal MF. Mitochondrial biology and oxidative stress in Parkinson disease pathogenesis. Nat Clin Pract Neurol 2008; 4(11): 600-9. [http://dx.doi.org/10.1038/ncpneuro0924] [PMID: 18978800] |
| [10] | Schapira AHV, Gegg M. Mitochondrial contribution to Parkinson’s disease pathogenesis. Parkinsons Dis 2011; 2011: 159160. [http://dx.doi.org/10.4061/2011/159160] |
| [11] | Feany MB, Bender WW. Drosophila model of Parkinson’s disease. Nature 2000; 404(6776): 394-8. [http://dx.doi.org/10.1038/35006074] [PMID: 10746727] |
| [12] | Bonini NM, Fortini ME. Human neurodegenerative disease modeling using Drosophila. Annu Rev Neurosci 2003; 26: 627-56. [http://dx.doi.org/10.1146/annurev.neuro.26.041002.131425] [PMID: 12704223] |
| [13] | Lu B, Vogel H. Drosophila models of neurodegenerative diseases. Annu Rev Pathol 2009; 4: 315-42. [http://dx.doi.org/10.1146/annurev.pathol.3.121806.151529] [PMID: 18842101] |
| [14] | Bonilla E, Medina-Leendertz S, Díaz S. Extension of life span and stress resistance of Drosophila melanogaster by long-term supplementation with melatonin. Exp Gerontol 2002; 37(5): 629-38. [http://dx.doi.org/10.1016/S0531-5565(01)00229-7] [PMID: 11909680] |
| [15] | Park JH, Jung JW, Ahn YJ, Kwon HW. Neuroprotective properties of phytochemicals against paraquat-induced oxidative stress and neurotoxicity in Drosophila melanogaster. Pestic Biochem Physiol 2012; 104: 118-25. [http://dx.doi.org/10.1016/j.pestbp.2012.07.006] |
| [16] | Hosamani R. Prophylactic treatment with Bacopa monnieri leaf powder mitigates paraquat-induced oxidative perturbations and lethality in Drosophila melanogaster. Indian J Biochem Biophys 2010; 47(2): 75-82. [PMID: 20521619] |
| [17] | Navarro JA, Heßner S, Yenisetti SC, et al. Analysis of dopaminergic neuronal dysfunction in genetic and toxin-induced models of Parkinson’s disease in Drosophila. J Neurochem 2014; 131(3): 369-82. [http://dx.doi.org/10.1111/jnc.12818] [PMID: 25040725] |
| [18] | Niveditha S, Ramesh SR, Shivanandappa T. Paraquat-induced movement disorder in relation to neurodegeneration in the brain of Drosophila melanogaster. Neurochem Res 2017; 42(11): 3310-20. [http://dx.doi.org/10.1007/s11064-017-2373-y] [PMID: 28819888] |
| [19] | Kim SI, Jung JW, Ahn YJ, Restifo LL, Kwon HW. Drosophila as a model system for studying lifespan and neuroprotective activities of plant-derived compounds. J Asia Pac Entomol 2011; 14: 509-17. [http://dx.doi.org/10.1016/j.aspen.2011.07.001] |
| [20] | Melov S. Animal models of oxidative stress, aging, and therapeutic antioxidant interventions. Int J Biochem Cell Biol 2002; 34(11): 1395-400. [http://dx.doi.org/10.1016/S1357-2725(02)00086-9] [PMID: 12200034] |
| [21] | Pandey KB, Rizvi SI. Plant polyphenols as dietary antioxidants in human health and disease. Oxid Med Cell Longev 2009; 2(5): 270-8. [http://dx.doi.org/10.4161/oxim.2.5.9498] [PMID: 20716914] |
| [22] | Yacoubian TA, Standaert DG. Targets for neuroprotection in Parkinson’s disease. Biochim Biophys Acta 2009; 1792: 676-87. |
| [23] | Lv C, Hong T, Yang Z, et al. Effect of Quercetin in the 1-Methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine-Induced mouse model of Parkinson’s Disease. Evid-Based Complement Alternat Med 2012; 1-6. 2012 |
| [24] | Dolatabadi EN, Mokhtarzadeh A, Ghareghoran SM, Dehghan G. Synthesis, characterization and antioxidant property of Quercetin-Tb(III) complex. Adv Pharm Bull 2014; 4(2): 101-4. [PMID: 24511472] |
| [25] | Sabogal-Guáqueta AM, Muñoz-Manco JI, Ramírez-Pineda JR, Lamprea-Rodriguez M, Osorio E, Cardona-Gómez GP. The flavonoid quercetin ameliorates Alzheimer’s disease pathology and protects cognitive and emotional function in aged triple transgenic Alzheimer’s disease model mice. Neuropharmacology 2015; 93: 134-45. [http://dx.doi.org/10.1016/j.neuropharm.2015.01.027] [PMID: 25666032] |
| [26] | Kirsch M, De Groot H. NAD(P)H, a directly operating antioxidant? FASEB J 2001; 15(9): 1569-74. [http://dx.doi.org/10.1096/fj.00-0823hyp] [PMID: 11427489] |
| [27] | Anderson DW, Bradbury KA, Schneider JS. Neuroprotection in Parkinson models varies with toxin administration protocol. Eur J Neurosci 2006; 24(11): 3174-82. [http://dx.doi.org/10.1111/j.1460-9568.2006.05192.x] [PMID: 17156378] |
| [28] | Jia H, Li X, Gao H, et al. High doses of nicotinamide prevent oxidative mitochondrial dysfunction in a cellular model and improve motor deficit in a Drosophila model of Parkinson’s disease. J Neurosci Res 2008; 86(9): 2083-90. [http://dx.doi.org/10.1002/jnr.21650] [PMID: 18381761] |
| [29] | Srivastava A, Harish SR, Shivanandappa T. Antioxidant activity of the roots of Decalepis hamiltonii (Wight & Arn.). Food Sci Technol 2006; 39: 1059-65. |
| [30] | Harish R, Divakar S, Srivastava A, Shivanandappa T. Isolation of antioxidant compounds from the methanolic extract of the roots of Decalepis hamiltonii (Wight and Arn.). J Agric Food Chem 2005; 53(20): 7709-14. [http://dx.doi.org/10.1021/jf051047c] [PMID: 16190621] |
| [31] | Srivastava A, Harish R, Shivanandappa T. Novel antioxidant compounds from the aqueous extract of the roots of Decalepis hamiltonii (Wight and Arn.) and their inhibitory effect on low-density lipoprotein oxidation. J Agric Food Chem 2006; 54(3): 790-5. [http://dx.doi.org/10.1021/jf052433x] [PMID: 16448184] |
| [32] | Srivastava A, Rao LJ, Shivanandappa T. Isolation of ellagic acid from the aqueous extract of the roots of Decalepis hamiltonii: Antioxidant activity and cytoprotective effect. Food Chem 2007; 103: 224-33. [http://dx.doi.org/10.1016/j.foodchem.2006.08.010] |
| [33] | Srivastava A, Rao LJ, Shivanandappa T. 4-(2-Hydroxypropan-2-yl)-1-methylcyclohexane-1,2-diol prevents xenobiotic induced cytotoxicity. Toxicol. In Vitro 2012; 26(6): 1040-6. [http://dx.doi.org/10.1016/j.tiv.2012.04.005] [PMID: 22531228] |
| [34] | Srivastava A, Rao LJ, Shivanandappa T. A novel cytoprotective antioxidant: Hydroxyisophthalic acid. Food Chem 2012; 132: 195965. [http://dx.doi.org/10.1016/j.foodchem.2011.12.032] |
| [35] | Srivastava A, Rao LJ, Shivanandappa T. 14-aminotetradecanoic acid exhibits antioxidant activity and ameliorates xenobiotics-induced cytotoxicity. Mol Cell Biochem 2012; 364(1-2): 1-9. [http://dx.doi.org/10.1007/s11010-011-1196-4] [PMID: 22198290] |
| [36] | Srivastava A, Shivanandappa T. Hepatoprotective effect of the aqueous extract of the roots of Decalepis hamiltonii against ethanol-induced oxidative stress in rats. Hepatol Res 2006; 35(4): 267-75. [http://dx.doi.org/10.1016/j.hepres.2006.04.011] [PMID: 16777477] |
| [37] | Srivastava A, Shivanandappa T. Decalepis hamiltonii roots boost antioxidant status of rat liver and brain. J Sci Food Agric 2009; 89: 2461-6. [http://dx.doi.org/10.1002/jsfa.3748] |
| [38] | Srivastava A, Shivanandappa T. Neuroprotective effect of Decalepis hamiltonii roots against ethanol-induced oxidative stress. Food Chem 2010; 119: 626-9. [http://dx.doi.org/10.1016/j.foodchem.2009.07.003] |
| [39] | Srivastava A, Shivanandappa T. Differential cholinesterase inhibition in the rat brain regions by dichlorvos and protective effect of Decalepis hamiltonii roots. Neurotoxicology 2011; 32(6): 931-4. [http://dx.doi.org/10.1016/j.neuro.2011.04.007] [PMID: 21571001] |
| [40] | Dolatshahi M, Farbood Y, Sarkaki A, Mansouri SM, Khodadadi A. Ellagic acid improves hyperalgesia and cognitive deficiency in 6-hydroxidopamine induced rat model of Parkinson’s disease. Iran J Basic Med Sci 2015; 18(1): 38-46. [PMID: 25810874] |
| [41] | Farbood Y, Sarkaki A, Dolatshahi M, Taqhi Mansouri SM, Khodadadi A. Ellagic acid protects the brain against 6-Hydroxydopamine induced neuroinflammation in a rat model of Parkinson’s Disease. Basic Clin Neurosci 2015; 6(2): 83-9. [PMID: 27307952] |
| [42] | Ahmed T, Setzer WN, Nabavi SF, et al. Insights into effects of ellagic acid on the nervous system: A mini review. Curr Pharm Des 2016; 22(10): 1350-60. [http://dx.doi.org/10.2174/1381612822666160125114503] [PMID: 26806345] |
| [43] | Haddadi M, Jahromi SR, Nongthomba U, Shivanandappa T, Ramesh SR. 4-Hydroxyisophthalic acid from Decalepis hamiltonii rescues the neurobehavioral deficit in transgenic Drosophila model of taupathies. Neurochem Int 2016; 100: 78-90. [http://dx.doi.org/10.1016/j.neuint.2016.09.007] [PMID: 27615061] |
| [44] | Rzezniczak TZ, Douglas LA, Watterson JH, Merritt TJ. Paraquat administration in Drosophila for use in metabolic studies of oxidative stress. Anal Biochem 2011; 419(2): 345-7. [http://dx.doi.org/10.1016/j.ab.2011.08.023] [PMID: 21910964] |
| [45] | LeBel CP, Ischiropoulos H, Bondy SC. Evaluation of the probe 2′,7′-dichlorofluorescin as an indicator of reactive oxygen species formation and oxidative stress. Chem Res Toxicol 1992; 5(2): 227-31. [http://dx.doi.org/10.1021/tx00026a012] [PMID: 1322737] |
| [46] | Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 1979; 95(2): 351-8. [http://dx.doi.org/10.1016/0003-2697(79)90738-3] [PMID: 36810] |
| [47] | Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys 1959; 82(1): 70-7. [http://dx.doi.org/10.1016/0003-9861(59)90090-6] [PMID: 13650640] |
| [48] | Marklund S, Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem 1974; 47(3): 469-74. [http://dx.doi.org/10.1111/j.1432-1033.1974.tb03714.x] [PMID: 4215654] |
| [49] | Aebi H. Catalase.Methods of Enzymatic Analysis 3rd ed. 1983; 273-86. |
| [50] | Ellman GL, Courtney KD, Andres V Jr, Feather-Stone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 1961; 7: 88-95. [http://dx.doi.org/10.1016/0006-2952(61)90145-9] [PMID: 13726518] |
| [51] | Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem 1951; 193(1): 265-75. [PMID: 14907713] |
| [52] | Ishige K, Schubert D, Sagara Y. Flavonoids protect neuronal cells from oxidative stress by three distinct mechanisms. Free Radic Biol Med 2001; 30(4): 433-46. [http://dx.doi.org/10.1016/S0891-5849(00)00498-6] [PMID: 11182299] |
| [53] | Kanski J, Aksenova M, Stoyanova A, Butterfield DA. Ferulic acid antioxidant protection against hydroxyl and peroxyl radical oxidation in synaptosomal and neuronal cell culture systems in vitro: Structure-activity studies. J Nutr Biochem 2002; 13(5): 273-81. [http://dx.doi.org/10.1016/S0955-2863(01)00215-7] [PMID: 12015157] |
| [54] | Haddadi M, Jahromi SR, Shivanandappa T, Ramesh SR. Decalepis hamiltonii root extract attenuates the age-related decline in the cognitive function in Drosophila melanogaster. Behav Brain Res 2013; 249: 8-14. [http://dx.doi.org/10.1016/j.bbr.2013.04.017] [PMID: 23608486] |
| [55] | Jahromi SR, Haddadi M, Shivanandappa T, Ramesh SR. Neuroprotective effect of Decalepis hamiltonii in paraquat-induced neurotoxicity in Drosophila melanogaster: Biochemical and behavioral evidences. Neurochem Res 2013; 38(12): 2616-24. [http://dx.doi.org/10.1007/s11064-013-1179-9] [PMID: 24173775] |
| [56] | Jahromi SR, Haddadi M, Shivanandappa T, Ramesh SR. Attenuation of neuromotor deficits by natural antioxidants of Decalepis hamiltonii in transgenic Drosophila model of Parkinson’s disease. Neuroscience 2015; 293: 136-50. [http://dx.doi.org/10.1016/j.neuroscience.2015.02.048] [PMID: 25754960] |
| [57] | Abdalla FH, Schmatz R, Cardoso AM, et al. Quercetin protects the impairment of memory and anxiogenic-like behavior in rats exposed to cadmium: Possible involvement of the acetylcholinesterase and Na(+),K(+)-ATPase activities. Physiol Behav 2014; 135: 152-67. [http://dx.doi.org/10.1016/j.physbeh.2014.06.008] [PMID: 24952260] |
| [58] | Maiese K, Chong ZZ. Nicotinamide: necessary nutrient emerges as a novel cytoprotectant for the brain. Trends Pharmacol Sci 2003; 24(5): 228-32. [http://dx.doi.org/10.1016/S0165-6147(03)00078-6] [PMID: 12767721] |
| [59] | Mandir AS, Przedborski S, Jackson-Lewis V, et al. Poly(ADP-ribose) polymerase activation mediates 1-methyl-4-phenyl-1, 2,3,6-tetrahydropyridine (MPTP)-induced parkinsonism. Proc Natl Acad Sci USA 1999; 96(10): 5774-9. [http://dx.doi.org/10.1073/pnas.96.10.5774] [PMID: 10318960] |
| [60] | Anderson DW, Bradbury KA, Schneider JS. Broad neuroprotective profile of nicotinamide in different mouse models of MPTP-induced parkinsonism. Eur J Neurosci 2008; 28(3): 610-7. [http://dx.doi.org/10.1111/j.1460-9568.2008.06356.x] [PMID: 18702732] |
| [61] | Santos L, Escande C, Denicola A. Potential modulation of sirtuins by oxidative stress. Oxid Med Cell Longev 2016; 2016: 9831825. [http://dx.doi.org/10.1155/2016/9831825] |
| [62] | Imai S, Guarente L. It takes two to tango: NAD(+) and sirtuins in aging/longevity control. NPJ Aging Mech Dis 2016; 2: 16017. [http://dx.doi.org/10.1038/npjamd.2016.17] [PMID: 28721271] |
| [63] | Costa LG, Garrick JM, Roquè PJ, Pellacani C, Pellacani C. Mechanisms of neuroprotection by quercetin: counteracting oxidative stress and more. Oxid Med Cell Longev 2016; 2016: 1-10. [http://dx.doi.org/10.1155/2016/2986796] |
| [64] | Arredondo F, Echeverry C, Abin-Carriquiry JA, et al. After cellular internalization, quercetin causes Nrf2 nuclear translocation, increases glutathione levels, and prevents neuronal death against an oxidative insult. Free Radic Biol Med 2010; 49(5): 738-47. [http://dx.doi.org/10.1016/j.freeradbiomed.2010.05.020] [PMID: 20554019] |
| [65] | Wang DM, Li SQ, Wu WL, Zhu XY, Wang Y, Yuan H-Y. Effects of long-term treatment with quercetin on cognition and mitochondrial function in a mouse model of Alzheimer’s disease. Neurochem Res 2014; 39(8): 1533-43. [http://dx.doi.org/10.1007/s11064-014-1343-x] [PMID: 24893798] |
| [66] | Moreira PI, Zhu X, Wang X, et al. Mitochondria: A therapeutic target in neurodegeneration. Biochim Biophys Acta - Mol Basis Dis 2010; 1802: 212-0. |
| [67] | Bose A, Beal MF. Mitochondrial dysfunction in Parkinson’s disease. J Neurochem 2016; 139(Suppl. 1): 216-31. [http://dx.doi.org/10.1111/jnc.13731] [PMID: 27546335] |











