- Home
- About Journals
-
Information for Authors/ReviewersEditorial Policies
Publication Fee
Publication Cycle - Process Flowchart
Online Manuscript Submission and Tracking System
Publishing Ethics and Rectitude
Authorship
Author Benefits
Reviewer Guidelines
Guest Editor Guidelines
Peer Review Workflow
Quick Track Option
Copyediting Services
Bentham Open Membership
Bentham Open Advisory Board
Archiving Policies
Fabricating and Stating False Information
Post Publication Discussions and Corrections
Editorial Management
Advertise With Us
Funding Agencies
Rate List
Kudos
General FAQs
Special Fee Waivers and Discounts
- Contact
- Help
- About Us
- Search

The Open Food Science Journal
(Discontinued)
ISSN: 1874-2564 ― Volume 13, 2021
The Influence of Lactobacilli in GABA and Amino Acid Profile of Fermented Mature Coconut Water
Izuddin Abdul Rahman, Mohd Izwan Mohd Lazim, Suhaiza Mohamad, Koh Soo Peng*, Muhammad Anas Othaman, Musaalbakri Abdul Manan, Mohd Azzammil Mohd Asri
Abstract
Objective:
Mature coconut water (MCW) is a waste product from the coconut milk industry. It is sour and unpalatable, yet it contains sufficient nutrients for microbial growth.
Methods:
Four Lactic Acid Bacteria (LAB), namely L. acidophilus B0258, L. brevis VM1, L. casei B0189, and L. plantarum B0103 were used to ferment MCW over 120 h. Among these LAB strains, only L. casei was capable to grow well with the highest viable bacteria count of 1 x 1011 colony forming unit (cfu)/ml. Although all LAB produced α-aminobutyric acid (GABA) after fermentation, L. acidophilus and L. plantarum produced the highest amount of GABA with the increment of 35.4%±7.9 and 38.9%±1.7, respectively. Other amino acid profiles of fermented MCW were also investigated, but most of them were consumed by the LAB. Both L. acidophilus and L. plantarum utilized the most essential amino acids. Within the first 24 h, GABA content was enhanced in all LAB strains when they were actively growing.
Result and Conclusion:
This study showed that both L. acidophilus and L. plantarum have great potentials to increase GABA content in MCW. Fermented coconut water can be formulated as a healthy functional drink as GABA is known to have therapeutic value in alleviating stress as reported by past research findings.
Article Information
Identifiers and Pagination:
Year: 2018Volume: 10
First Page: 8
Last Page: 15
Publisher Id: TOFSJ-10-8
DOI: 10.2174/1874256401810010008
Article History:
Received Date: 26/7/2018Revision Received Date: 8/8/2018
Acceptance Date: 18/9/2018
Electronic publication date: 17/10/2018
Collection year: 2018
open-access license: This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International Public License (CC-BY 4.0), a copy of which is available at: (https://creativecommons.org/licenses/by/4.0/legalcode). This license permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
* Address correspondence to this author at the Biotechnology and Nanotechnology Research Centre, MARDI Headquarters, 43400 Serdang, Selangor, Malaysia, Tel: +60389536097; E-mails: karenkoh@mardi.gov.my; karenkoh999@gmail.com
| Open Peer Review Details | |||
|---|---|---|---|
| Manuscript submitted on 26-7-2018 |
Original Manuscript | The Influence of Lactobacilli in GABA and Amino Acid Profile of Fermented Mature Coconut Water | |
1. INTRODUCTION
The demand for coconut water is increasing due to its unique and refreshing taste, rehydration potential [1Prades A, Dornier M, Diop N, Pain J-P. Coconut water preservation and processing: A review. Fruits 2012; 67(3): 157-71.
[http://dx.doi.org/10.1051/fruits/2012009] ], nutritional and health benefits [2Yong JW, Ge L, Ng YF, Tan SN. The chemical composition and biological properties of coconut (Cocos nucifera L.) water. Molecules 2009; 14(12): 5144-64.
[http://dx.doi.org/10.3390/molecules14125144] [PMID: 20032881] -4Campbell-Falck D, Thomas T, Falck TM, Tutuo N, Clem K. The intravenous use of coconut water. Am J Emerg Med 2000; 18(1): 108-11.
[http://dx.doi.org/10.1016/S0735-6757(00)90062-7] [PMID: 10674546] ]. Due to this high demand, sources for coconut water are more ubiquitous, and more land has being turned into coconut plantation. The coconut water for drinking is mostly taken from young coconuts, which have the most water. As the coconut mature, its water content decreases and turns sour, and eventually become less palatable for fresh consumption. Nevertheless, the natural aging process of coconut water produced other health-benefiting bioactive compounds, such as cytokinins [5Mohd Lazim MI, Badruzaman NA, Koh SP, Long K. Quantification of cytokinins in coconut water from different maturation stages of malaysias coconut (Cocos nucifera L.) varieties. J Food Process Eng 2015; 6(11)].
Currently, the mature coconut is being used for the production of desiccated coconut product and coconut milk, and its water is wasted. Besides containing numerous compounds vital for human health, the water contains enough nutrients to support microbial growth. Lactic acid bacteria (LAB) can be used to ferment mature coconut water (MCW) and improve the bioactive compounds. One of the bioactive compounds that are widely studied is γ-aminobutyric acid (GABA), which is a by-product of several microbial fermentations including LAB [6Dhakal R, Bajpai VK, Baek K-H. Production of gaba (γ - Aminobutyric acid) by microorganisms: A review. Braz J Microbiol 2012; 43(4): 1230-41.
[http://dx.doi.org/10.1590/S1517-83822012000400001] [PMID: 24031948] ]. GABA is the major inhibitory neurotransmitter that controls the central nervous system and plays a vital role in numerous human physiological processes. Abdou et al. conducted a study on human model reporting the function of GABA in promoting relaxation and improving body immunity during stress [7Abdou AM, Higashiguchi S, Horie K, Kim M, Hatta H, Yokogoshi H. Relaxation and immunity enhancement effects of γ-aminobutyric acid (GABA) administration in humans. Biofactors 2006; 26(3): 201-8.
[http://dx.doi.org/10.1002/biof.5520260305] [PMID: 16971751] ].
The LAB produce GABA in response to stress conditions, such as acidic environment. The presence of glutamate decarboxylase (GAD) system allows LAB to survive in low pH environment by producing GABA, which consumes protons they produced through fermentation to maintain pH homeostasis [8Feehily C, Karatzas KAG. Role of glutamate metabolism in bacterial responses towards acid and other stresses. J Appl Microbiol 2013; 114(1): 11-24.
[http://dx.doi.org/10.1111/j.1365-2672.2012.05434.x] [PMID: 22924898] ]. Most of the LAB strains also lack cell envelope proteinases, limiting their growth in protein-rich foods; therefore, LAB such as L. brevis have been shown to produce high amount of GABA as a strategy to grow in media with high protein content [9Wu Q, Shah NP. High γ-aminobutyric acid production from lactic acid bacteria: Emphasis on Lactobacillus brevis as a functional dairy starter. Crit Rev Food Sci Nutr 2017; 57(17): 3661-72.
[http://dx.doi.org/10.1080/10408398.2016.1147418] [PMID: 26980301] ]. LAB are widely used for the fermentation of food products, such as cheese, kimchi, sourdough, sauerkraut, yogurt, and many other fermented foods [10Gänzle MG. Lactic metabolism revisited: Metabolism of lactic acid bacteria in food fermentations and food spoilage. Curr Opin Food Sci 2015; 2: 106-17.
[http://dx.doi.org/10.1016/j.cofs.2015.03.001] ]. Many studies have reported high amount of naturally produced GABA when LAB were used in the fermentation of food products [11Ratanaburee A, Kantachote D, Charernjiratrakul W, Sukhoom A. Selection of γ-aminobutyric acid-producing lactic acid bacteria and their potential as probiotics for use as starter cultures in Thai fermented sausages (Nham). Int J Food Sci Technol 2013; 48(7): 1371-82.
[http://dx.doi.org/10.1111/ijfs.12098] , 12Cho SY, Park MJ, Kim KM, Ryu J-H, Park HJ. Production of high γ-aminobutyric acid (GABA) sour kimchi using lactic acid bacteria isolated from mukeunjee kimchi. Food Sci Biotechnol 2011; 20(2): 403-8.
[http://dx.doi.org/10.1007/s10068-011-0057-y] ].
Therefore, LAB strains are considered as the suitable candidate for enhancing GABA in the fermentation of MCW. The main objective of this study was to screen the potential of four locally isolated LAB strains in enhancing GABA content in MCW. The amino acid profile of the fermented MCW was also examined throughout 120 h of fermentation. The results of this study allow us to formulate a fermented functional drink from MCW with naturally produced GABA by the selected LAB strains.
2. MATERIAL AND METHODS
2.1. Coconut Water Preparation
Fresh coconut water was obtained from mature coconut fruits (Cocos nucifera L.), which were harvested from the Malaysian Agricultural and Research Development Institute (MARDI) coconut plantation (Perak, Malaysia). The coconuts were thoroughly disinfected with alcohol to reduce the risk of contamination before collecting the coconut water. A pasteurization condition study was conducted previously on MCW, which took into account the sterility of the MCW before fermentation and the preservation of the existing bioactive compounds of interest in MCW. Based on that study, the coconut water was pasteurized at 105°C for 10 min before being subjected to microbial fermentation.
2.2. Bacterial Strains and Culturing
The starter culture was prepared according to a study with modifications [13Kantachote D, Ratanaburee A, Hayisama-ae W, Sukhoom A, Nunkaew T. The use of potential probiotic Lactobacillus plantarum DW12 for producing a novel functional beverage from mature coconut water. J Funct Foods 2017; 32: 401-8.
[http://dx.doi.org/10.1016/j.jff.2017.03.018] ]. Four locally isolated LAB strains, namely Lactobacillus acidophilus B0258, Lactobacillus brevis VM1, Lactobacillus casei B0189 and Lactobacillus plantarum B0103, were obtained from MARDI’s Centre of Functional Food Cultures (CFFC). Fresh starter culture of each strain was prepared on de Man Rogosa and Sharp (MRS) medium and incubated at 37°C for 24 h. After 24 h, a single loop of pure culture colony of each LAB strains was inoculated into 15 mL of coconut water and incubated at 37 °C for 24 h, which was then used as inoculum stock.
2.3. Fermentation
The pasteurized coconut water was inoculated with 3% (v/v) starter culture and incubated at 37°C under static condition for 5 days. The growth profile of each LAB strain was observed from colony forming unit (cfu)/ml, and the pH of the media was determined at an interval time of 24 h.
2.4. Quantitative Analysis
Amino acid and GABA contents were analyzed using ultra performance liquid chromatography (UPLC) according to a method described by Danial et al. with slight modifications [14Danial AM, Peng KS, Long K. Enrichment of mung bean with L-DOPA, GABA, essential amino acids via controlled biofermentation strategy. Int J Biotechnol Wellness Ind 2015; 4: 114-22.]. Briefly, 10 µL of sample was derivatized using 70 µL of AccQ-Tag™ Ultra and completely dissolved in borate buffer. A total of 20 µL of AccQ-Tag™ Fluor agent was added, and the mixture was heated to 55 °C for 10 min. A volume of 1 µL mixture solution was injected into the UPLC system. An AccQ-Tag™ Ultra column (2.1 mm x 100 mm, 1.7 µm) was used with a flow rate of 0.7 mL/min. The column temperature was set to 55 °C and the UV spectra were read at 260 nm. The elution solvents consisted of mobile phase A: ACN:formic acid:ammonium (10:6:84) and mobile phase B: ACN:formic acid (98:2) were prepared. The bi-elution strategy was performed as follows: 99.9% A (0.01% B) was maintained from 0.00 to 0.54 min; followed by linear gradient of A from 99.9 to 90.9% at 0.54 to 5.74 min; linear gradient of A from 90.9 to 78.8% at 5.74 to 7.74 min; linear gradient of A from 78.8 to 40.4% from 7.74 to 8.50 min; constant flow of A at 40.4% for 0.30 min; linear gradient of A from 40.4 to 99.9% at 8.80 to 8.90 min; and finally a constant flow of A at 99.9% for 2.10 min. The amino acid content was quantified from the standard curve of alanine, arginine, aspartic acid, cysteine, glutamic acid, glycine, histidine, isoleucine, leucine, lysine, methionine, phenylalanine, proline, serine, threonine, tryptophan, tyrosine, valine. The total essential amino acids were calculated based on the sum of histidine, phenylalanine, threonine, methionine, leucine, isoleucine, tryptophan, lysine and valine. All analyses were performed in triplicate.
3. RESULTS AND DISCUSSION
3.1. Bacterial Growth
The advantage of LAB in the coconut water fermentation is their ability to survive at low pH environment. Fig. (1A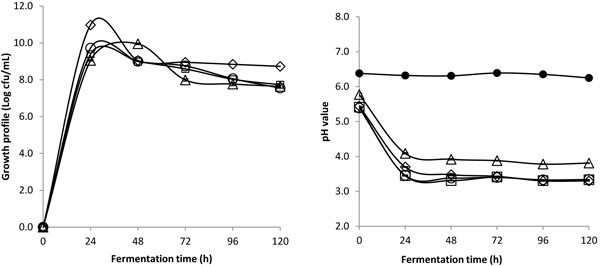 ) shows that all LAB strains were able to reach their viable counts above 109 cfu/ml after 24 h of fermentation but later, showed a slight decline in the bacterial viability rate after 5 days of fermentation. However, the viable count of all LAB strains still remained above 7.5 (log10 cfu/ml). Among the four selected LAB strains, only L. casei was observed to be actively growing within the first 24 h with the highest bacterial viable count of 1011 cfu/ml. However, the viability rate of L. casei declined significantly after 48 h and remained constant in its growth profile throughout 5 days fermentation time. The slight decreased in the bacterial viability rate might be caused by the highly acidic growth media condition; thus, retarding the rapid growth of the bacteria [15Barla F, Koyanagi T, Tokuda N, et al. The γ-aminobutyric acid-producing ability under low pH conditions of lactic acid bacteria isolated from traditional fermented foods of Ishikawa Prefecture, Japan, with a strong ability to produce ACE-inhibitory peptides. Biotechnol Rep (Amst) 2016; 10: 105-10.
) shows that all LAB strains were able to reach their viable counts above 109 cfu/ml after 24 h of fermentation but later, showed a slight decline in the bacterial viability rate after 5 days of fermentation. However, the viable count of all LAB strains still remained above 7.5 (log10 cfu/ml). Among the four selected LAB strains, only L. casei was observed to be actively growing within the first 24 h with the highest bacterial viable count of 1011 cfu/ml. However, the viability rate of L. casei declined significantly after 48 h and remained constant in its growth profile throughout 5 days fermentation time. The slight decreased in the bacterial viability rate might be caused by the highly acidic growth media condition; thus, retarding the rapid growth of the bacteria [15Barla F, Koyanagi T, Tokuda N, et al. The γ-aminobutyric acid-producing ability under low pH conditions of lactic acid bacteria isolated from traditional fermented foods of Ishikawa Prefecture, Japan, with a strong ability to produce ACE-inhibitory peptides. Biotechnol Rep (Amst) 2016; 10: 105-10.
[http://dx.doi.org/10.1016/j.btre.2016.04.002] [PMID: 28352530] ].
Fig. (1B ) shows a dramatic decline in pH in all fermented coconut water samples after 24 h of incubation and remained constant throughout 5 days of fermentation period. Among all LAB strains, only coconut water fermented with L. brevis showed slightly higher pH value of 3.8 even though subjected to prolonged fermentation up to 5 days. A very low, acidic pH condition might cause a decrease in bacterial viability rate. Nevertheless, the viability rate of L. brevis showed a slight decline after day 3 of fermentation Fig. (1A
) shows a dramatic decline in pH in all fermented coconut water samples after 24 h of incubation and remained constant throughout 5 days of fermentation period. Among all LAB strains, only coconut water fermented with L. brevis showed slightly higher pH value of 3.8 even though subjected to prolonged fermentation up to 5 days. A very low, acidic pH condition might cause a decrease in bacterial viability rate. Nevertheless, the viability rate of L. brevis showed a slight decline after day 3 of fermentation Fig. (1A ). The higher pH was probably the reason why L. brevis was able to sustain its growth within 48 h. Moreover, many studies have reported L. brevis as the predominant microbe that can continue spoiling food despite the hostile conditions after 24 h of fermentations [16Zhao Y, Knøchel S, Siegumfeldt H. Heterogeneity between and within strains of Lactobacillus brevis exposed to beer compounds. Front Microbiol 2017; 8: 239.
). The higher pH was probably the reason why L. brevis was able to sustain its growth within 48 h. Moreover, many studies have reported L. brevis as the predominant microbe that can continue spoiling food despite the hostile conditions after 24 h of fermentations [16Zhao Y, Knøchel S, Siegumfeldt H. Heterogeneity between and within strains of Lactobacillus brevis exposed to beer compounds. Front Microbiol 2017; 8: 239.
[http://dx.doi.org/10.3389/fmicb.2017.00239] [PMID: 28261191] ]. Most of these studies showed the spoilage of beer by L. brevis, which was due to its hop resistance [17Behr J, Gänzle MG, Vogel RF. Characterization of a highly hop-resistant Lactobacillus brevis strain lacking hop transport. Appl Environ Microbiol 2006; 72(10): 6483-92.
[http://dx.doi.org/10.1128/AEM.00668-06] [PMID: 17021196] ].
3.2. GABA Production
At the interval time of 24 h, all coconut water samples fermented with four LAB strains were collected to examine their amino acids profile throughout 5 days of fermentation as shown in Fig. (2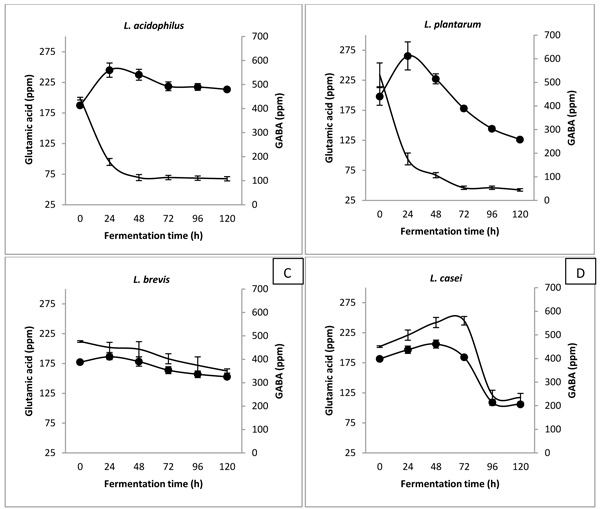 ). Generally, L. acidophilus, L. plantarum, L. brevis and L. casei showed an improvement in GABA content with increments of 35.4%±7.9, 38.9%±1.7, 5.8%±3.1 and 9.7%±2.9, respectively, after 24 h of fermentation. It is well known that the major pathway for GABA production involves the utilization of glutamic acid and pyridoxal 5’–phosphate (PLP) as its co-factor [18Petroff OAC. GABA and glutamate in the human brain. Neuroscientist 2002; 8(6): 562-73.
). Generally, L. acidophilus, L. plantarum, L. brevis and L. casei showed an improvement in GABA content with increments of 35.4%±7.9, 38.9%±1.7, 5.8%±3.1 and 9.7%±2.9, respectively, after 24 h of fermentation. It is well known that the major pathway for GABA production involves the utilization of glutamic acid and pyridoxal 5’–phosphate (PLP) as its co-factor [18Petroff OAC. GABA and glutamate in the human brain. Neuroscientist 2002; 8(6): 562-73.
[http://dx.doi.org/10.1177/1073858402238515] [PMID: 12467378] ]. Therefore, L. acidophilus and L. plantarum strains showed an inverse trend of glutamic acid level, implying that these bacteria consumed glutamic acid to produce GABA during fermentation.
On the other hand, the glutamic acid content of both L. brevis and L. casei showed a parallel trend of GABA profile change, indicating that both LAB strains might produce glutamic acid during fermentation, and part of the glutamic acid produced was consumed to produce GABA when the fermented coconut water became acidic after 24 h. A fermentation study without the addition of L-MSG also showed similar result whereby glutamine was converted into glutamic acid during the later stage of fermentation. Eventually, the glutamic acid was converted into small amount of GABA [19Mazzoli R, Pessione E, Dufour M, et al. Glutamate-induced metabolic changes in Lactococcus lactis NCDO 2118 during GABA production: Combined transcriptomic and proteomic analysis. Amino Acids 2010; 39(3): 727-37.
[http://dx.doi.org/10.1007/s00726-010-0507-5] [PMID: 20174841] ], implying that both glutamic acid and GABA were being produced in small quantity.
When exposed to the prolonged fermentation period under extreme conditions, GABA was metabolized in fermented coconut water to sustain the LAB growth under stress condition, resulting in the decline trend of GABA content as observed in all fermented coconut water. Zhuang et. al. also hypothesized that after 48 h of fermentation, GABA transaminase activity had to be restored to replenish pyruvate within the bacterial cells, leading to conversion of GABA to succinate semialdehyde and finally to succinate before entering the citric cycle [20Zhuang K, Jiang Y, Feng X, et al. Transcriptomic response to GABA-producing Lactobacillus plantarum CGMCC 1.2437T induced by L-MSG. PLoS One 2018; 13(6): e0199021.
[http://dx.doi.org/10.1371/journal.pone.0199021] [PMID: 29894506] ].
3.3. Amino Acid Profiles
Table 1. summarizes the changes in amino acid profiles of all LAB strains at the interval time of 24 h over 5 days fermentation period. Generally, most of the essential amino acids (EAAs) were consumed significantly after 24 h, particularly for L. acidophilus and L. plantarum. It was worth noting that both L. acidophilus and L. plantarum exhibited similar pattern changes of EAAs metabolism. In contrast, L. brevis and L. casei did not consume EAAs as compared with the other two counterparts but still showed a decreasing trend of EAAs content. This finding was supported by past studies, which reported a reduction in EAAs after fermentation with LAB, specifically L. acidophilus [21R. PS, K. RT. Amino acid composition and nutritional implications of milk fermented by various lactic cultures. J Food Qual 1982; 5(3): 235-43.
[http://dx.doi.org/10.1111/j.1745-4557.1982.tb00746.x] ]. It was initially thought that the reduction in EAAs by L. acidophilus and L. plantarum was used for the production of GABA; however, it was learned that the major pathway for GABA production utilizes glutamic acid, which is a non-essential amino acid (NEAA) [18Petroff OAC. GABA and glutamate in the human brain. Neuroscientist 2002; 8(6): 562-73.
[http://dx.doi.org/10.1177/1073858402238515] [PMID: 12467378] ]. Among all LAB fermented coconut samples, coconut water fermented with L. brevis remained with the highest amount of total soluble amino acids, especially for essential amino acids. This finding indicated that L. brevis-fermented coconut water could provide more beneficial effect for human consumption, as our body cannot synthesize essential amino acids. Whereas, L.acidophilus–fermented coconut water exhibited the highest content of GABA, indicating a great potential for functional beverage marketed as anti-stress coconut beverage.
4. RESULTS
Among non-essential amino acids, it was noted that aspartic acid was heavily consumed by L. acidophilus and L. plantarum Fig. (3A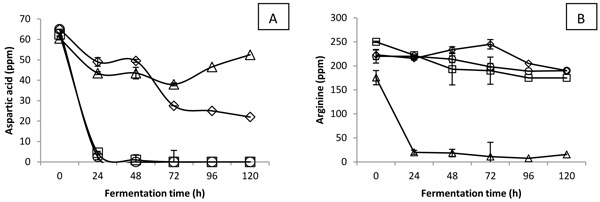 ). These fermented coconut water samples recorded higher increment in GABA content Fig. (2
). These fermented coconut water samples recorded higher increment in GABA content Fig. (2 ). It is unknown whether aspartic acid was consumed for the production of GABA or not. Nevertheless, aspartic acid was reported to be involved in stress response mechanism in LAB fermentation [22Papadimitriou K, Alegría Á, Bron PA, et al. Stress physiology of lactic acid bacteria. Microbiol Mol Biol Rev 2016; 80(3): 837-90.
). It is unknown whether aspartic acid was consumed for the production of GABA or not. Nevertheless, aspartic acid was reported to be involved in stress response mechanism in LAB fermentation [22Papadimitriou K, Alegría Á, Bron PA, et al. Stress physiology of lactic acid bacteria. Microbiol Mol Biol Rev 2016; 80(3): 837-90.
[http://dx.doi.org/10.1128/MMBR.00076-15] [PMID: 27466284] ]. The conversion of aspartic acid to alanine and carbon dioxide through enzyme aspartic acid decarboxylase (AspD) was demonstrated in L. acidophilus [23Altermann E, Russell WM, Azcarate-Peril MA, et al. Complete genome sequence of the probiotic lactic acid bacterium Lactobacillus acidophilus NCFM. Proc Natl Acad Sci USA 2005; 102(11): 3906-12.
[http://dx.doi.org/10.1073/pnas.0409188102] [PMID: 15671160] ] and L. plantarum [24Kleerebezem M, Boekhorst J, van Kranenburg R, et al. Complete genome sequence of Lactobacillus plantarum WCFS1. Proc Natl Acad Sci USA 2003; 100(4): 1990-5.
[http://dx.doi.org/10.1073/pnas.0337704100] [PMID: 12566566] ]. On the other hand, Fig. (3B ) shows a dramatic reduction in arginine content by L. brevis when compared to other strains of LAB. De Angelis’s study reported the involvement of arginine in adapting environmental stress through the arginine deiminase (ADI) pathway [25De Angelis M, Mariotti L, Rossi J, et al. Arginine catabolism by sourdough lactic acid bacteria: Purification and characterization of the arginine deiminase pathway enzymes from Lactobacillus sanfranciscensis CB1. Appl Environ Microbiol 2002; 68(12): 6193-201.
) shows a dramatic reduction in arginine content by L. brevis when compared to other strains of LAB. De Angelis’s study reported the involvement of arginine in adapting environmental stress through the arginine deiminase (ADI) pathway [25De Angelis M, Mariotti L, Rossi J, et al. Arginine catabolism by sourdough lactic acid bacteria: Purification and characterization of the arginine deiminase pathway enzymes from Lactobacillus sanfranciscensis CB1. Appl Environ Microbiol 2002; 68(12): 6193-201.
[http://dx.doi.org/10.1128/AEM.68.12.6193-6201.2002] [PMID: 12450844] ]. Enzyme ADI helps to generate ATP from arginine, allowing the LAB to cope with acidic stress condition. This phenomenon indicated that L brevis might consume arginine to adapt the stress of acidic environment, which displayed another stress response mechanism that was slightly different from other LAB strains. Based on the amino acid profile changes for L. casei-fermented coconut water, L. casei might utilize both glutamic acid and aspartic acids in its stress response system. Generally, it was concluded that different strains of LAB showed various trends of reductions in specific types of amino acids, indicating different stress response mechanisms in order to survive in acidic growth environment.
CONCLUSION
Most mature coconut water fermentation with different strains of LAB produced GABA as a stress response mechanism in surviving under extreme acidic environment. Among four LAB strains, both L. acidophilus and L. plantarum produced higher GABA content with the increment of 35.4% and 38.9%, respectively. On the other hand, L. brevis utilized arginine as its best strategy in coping with acidic stress growth environment. The main contributing role of these LAB fermentations was to enhance the nutrient of MCW, particularly GABA content. Therefore, both L. acidophilus and L. plantarum were selected for further functionality study.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
HUMAN AND ANIMAL RIGHTS
No Animals/Humans were used for studies that are base of this research.
CONSENT FOR PUBLICATION
Not applicable.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
This work was financially supported by the Development Fund (P-RB413-1001) of Malaysian Agricultural Research and Development Institute (MARDI) under the 11th Malaysia Plan.
REFERENCES
| [1] | Prades A, Dornier M, Diop N, Pain J-P. Coconut water preservation and processing: A review. Fruits 2012; 67(3): 157-71. [http://dx.doi.org/10.1051/fruits/2012009] |
| [2] | Yong JW, Ge L, Ng YF, Tan SN. The chemical composition and biological properties of coconut (Cocos nucifera L.) water. Molecules 2009; 14(12): 5144-64. [http://dx.doi.org/10.3390/molecules14125144] [PMID: 20032881] |
| [3] | DebMandal M, Mandal S. Coconut (Cocos nucifera L.: Arecaceae): In health promotion and disease prevention. Asian Pac J Trop Med 2011; 4(3): 241-7. [http://dx.doi.org/10.1016/S1995-7645(11)60078-3] [PMID: 21771462] |
| [4] | Campbell-Falck D, Thomas T, Falck TM, Tutuo N, Clem K. The intravenous use of coconut water. Am J Emerg Med 2000; 18(1): 108-11. [http://dx.doi.org/10.1016/S0735-6757(00)90062-7] [PMID: 10674546] |
| [5] | Mohd Lazim MI, Badruzaman NA, Koh SP, Long K. Quantification of cytokinins in coconut water from different maturation stages of malaysias coconut (Cocos nucifera L.) varieties. J Food Process Eng 2015; 6(11) |
| [6] | Dhakal R, Bajpai VK, Baek K-H. Production of gaba (γ - Aminobutyric acid) by microorganisms: A review. Braz J Microbiol 2012; 43(4): 1230-41. [http://dx.doi.org/10.1590/S1517-83822012000400001] [PMID: 24031948] |
| [7] | Abdou AM, Higashiguchi S, Horie K, Kim M, Hatta H, Yokogoshi H. Relaxation and immunity enhancement effects of γ-aminobutyric acid (GABA) administration in humans. Biofactors 2006; 26(3): 201-8. [http://dx.doi.org/10.1002/biof.5520260305] [PMID: 16971751] |
| [8] | Feehily C, Karatzas KAG. Role of glutamate metabolism in bacterial responses towards acid and other stresses. J Appl Microbiol 2013; 114(1): 11-24. [http://dx.doi.org/10.1111/j.1365-2672.2012.05434.x] [PMID: 22924898] |
| [9] | Wu Q, Shah NP. High γ-aminobutyric acid production from lactic acid bacteria: Emphasis on Lactobacillus brevis as a functional dairy starter. Crit Rev Food Sci Nutr 2017; 57(17): 3661-72. [http://dx.doi.org/10.1080/10408398.2016.1147418] [PMID: 26980301] |
| [10] | Gänzle MG. Lactic metabolism revisited: Metabolism of lactic acid bacteria in food fermentations and food spoilage. Curr Opin Food Sci 2015; 2: 106-17. [http://dx.doi.org/10.1016/j.cofs.2015.03.001] |
| [11] | Ratanaburee A, Kantachote D, Charernjiratrakul W, Sukhoom A. Selection of γ-aminobutyric acid-producing lactic acid bacteria and their potential as probiotics for use as starter cultures in Thai fermented sausages (Nham). Int J Food Sci Technol 2013; 48(7): 1371-82. [http://dx.doi.org/10.1111/ijfs.12098] |
| [12] | Cho SY, Park MJ, Kim KM, Ryu J-H, Park HJ. Production of high γ-aminobutyric acid (GABA) sour kimchi using lactic acid bacteria isolated from mukeunjee kimchi. Food Sci Biotechnol 2011; 20(2): 403-8. [http://dx.doi.org/10.1007/s10068-011-0057-y] |
| [13] | Kantachote D, Ratanaburee A, Hayisama-ae W, Sukhoom A, Nunkaew T. The use of potential probiotic Lactobacillus plantarum DW12 for producing a novel functional beverage from mature coconut water. J Funct Foods 2017; 32: 401-8. [http://dx.doi.org/10.1016/j.jff.2017.03.018] |
| [14] | Danial AM, Peng KS, Long K. Enrichment of mung bean with L-DOPA, GABA, essential amino acids via controlled biofermentation strategy. Int J Biotechnol Wellness Ind 2015; 4: 114-22. |
| [15] | Barla F, Koyanagi T, Tokuda N, et al. The γ-aminobutyric acid-producing ability under low pH conditions of lactic acid bacteria isolated from traditional fermented foods of Ishikawa Prefecture, Japan, with a strong ability to produce ACE-inhibitory peptides. Biotechnol Rep (Amst) 2016; 10: 105-10. [http://dx.doi.org/10.1016/j.btre.2016.04.002] [PMID: 28352530] |
| [16] | Zhao Y, Knøchel S, Siegumfeldt H. Heterogeneity between and within strains of Lactobacillus brevis exposed to beer compounds. Front Microbiol 2017; 8: 239. [http://dx.doi.org/10.3389/fmicb.2017.00239] [PMID: 28261191] |
| [17] | Behr J, Gänzle MG, Vogel RF. Characterization of a highly hop-resistant Lactobacillus brevis strain lacking hop transport. Appl Environ Microbiol 2006; 72(10): 6483-92. [http://dx.doi.org/10.1128/AEM.00668-06] [PMID: 17021196] |
| [18] | Petroff OAC. GABA and glutamate in the human brain. Neuroscientist 2002; 8(6): 562-73. [http://dx.doi.org/10.1177/1073858402238515] [PMID: 12467378] |
| [19] | Mazzoli R, Pessione E, Dufour M, et al. Glutamate-induced metabolic changes in Lactococcus lactis NCDO 2118 during GABA production: Combined transcriptomic and proteomic analysis. Amino Acids 2010; 39(3): 727-37. [http://dx.doi.org/10.1007/s00726-010-0507-5] [PMID: 20174841] |
| [20] | Zhuang K, Jiang Y, Feng X, et al. Transcriptomic response to GABA-producing Lactobacillus plantarum CGMCC 1.2437T induced by L-MSG. PLoS One 2018; 13(6): e0199021. [http://dx.doi.org/10.1371/journal.pone.0199021] [PMID: 29894506] |
| [21] | R. PS, K. RT. Amino acid composition and nutritional implications of milk fermented by various lactic cultures. J Food Qual 1982; 5(3): 235-43. [http://dx.doi.org/10.1111/j.1745-4557.1982.tb00746.x] |
| [22] | Papadimitriou K, Alegría Á, Bron PA, et al. Stress physiology of lactic acid bacteria. Microbiol Mol Biol Rev 2016; 80(3): 837-90. [http://dx.doi.org/10.1128/MMBR.00076-15] [PMID: 27466284] |
| [23] | Altermann E, Russell WM, Azcarate-Peril MA, et al. Complete genome sequence of the probiotic lactic acid bacterium Lactobacillus acidophilus NCFM. Proc Natl Acad Sci USA 2005; 102(11): 3906-12. [http://dx.doi.org/10.1073/pnas.0409188102] [PMID: 15671160] |
| [24] | Kleerebezem M, Boekhorst J, van Kranenburg R, et al. Complete genome sequence of Lactobacillus plantarum WCFS1. Proc Natl Acad Sci USA 2003; 100(4): 1990-5. [http://dx.doi.org/10.1073/pnas.0337704100] [PMID: 12566566] |
| [25] | De Angelis M, Mariotti L, Rossi J, et al. Arginine catabolism by sourdough lactic acid bacteria: Purification and characterization of the arginine deiminase pathway enzymes from Lactobacillus sanfranciscensis CB1. Appl Environ Microbiol 2002; 68(12): 6193-201. [http://dx.doi.org/10.1128/AEM.68.12.6193-6201.2002] [PMID: 12450844] |