- Home
- About Journals
-
Information for Authors/ReviewersEditorial Policies
Publication Fee
Publication Cycle - Process Flowchart
Online Manuscript Submission and Tracking System
Publishing Ethics and Rectitude
Authorship
Author Benefits
Reviewer Guidelines
Guest Editor Guidelines
Peer Review Workflow
Quick Track Option
Copyediting Services
Bentham Open Membership
Bentham Open Advisory Board
Archiving Policies
Fabricating and Stating False Information
Post Publication Discussions and Corrections
Editorial Management
Advertise With Us
Funding Agencies
Rate List
Kudos
General FAQs
Special Fee Waivers and Discounts
- Contact
- Help
- About Us
- Search

The Open Food Science Journal
(Discontinued)
ISSN: 1874-2564 ― Volume 13, 2021
Study of Acidified Aqueous Extraction of Phenolic Compounds from Hibiscus sabdariffa L. calyces
Alessandra Piovesana, Caciano P. Zapata Noreña*
Abstract
Introduction:
Hibiscus calyces are important sources from anthocyanins and pigments. The recovery of these bioactive compounds using non-organic solvents becomes very attractive for the food industry.
Methods:
For this reason, the separation of phenolic compounds by acidified aqueous extraction from hibiscus calyces was studied. The experiments were conducted by a fractional factorial design.
Result and Conclusion:
Four factors were evaluated: temperature, time, stirring speed and enzyme concentration. The extracts produced were subjected to analysis of color (L*, a*, b* and Chroma), total monomeric anthocyanins, antioxidant capacity by ABTS and fourteen phenolic compounds were quantified. The results showed that the best condition to obtain hibiscus calyces extract was using an enzyme concentration of 50 µL/1000 g hibiscus extract, 400 rpm of stirring speed at 55 ºC by 4 hours of extraction, that corresponded to concentrations of 17595, 7516, 2568 μg/g, expressed on a dry basis, for total phenolic compounds, delphinidin 3-sambubioside and cyanidin 3-sambubioside, respectively, and antioxidant capacity measured by ABTS of 7.8 µmol of Trolox equivalent per gram.
Article Information
Identifiers and Pagination:
Year: 2019Volume: 11
First Page: 25
Last Page: 34
Publisher Id: TOFSJ-11-25
DOI: 10.2174/1874256401911010025
Article History:
Received Date: 27/9/2018Revision Received Date: 24/1/2019
Acceptance Date: 31/1/2019
Electronic publication date: 28/02/2019
Collection year: 2019
open-access license: This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International Public License (CC-BY 4.0), a copy of which is available at: https://creativecommons.org/licenses/by/4.0/legalcode. This license permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
* Address correspondence to this author at the Institute of Food Science and Technology, Federal University of Rio Grande do Sul, Av. Bento Gonçalves, 9500, CEP 91501-970, Porto Alegre, RS, Brazil; Tel: +55-51-33086673; Fax: +55-51-33087048; E-mail: czapatan@ufrgs.br
| Open Peer Review Details | |||
|---|---|---|---|
| Manuscript submitted on 27-9-2018 |
Original Manuscript | Study of Acidified Aqueous Extraction of Phenolic Compounds from Hibiscus sabdariffa L. calyces | |
1. INTRODUCTION
Hibiscus is a native plant from Africa and Asia, belonging to Malvaceae family. Depicted as a bushy and branched plant, with a height of up to 2.5 m, of the purplish stem with green-purple leaves, whose solitary flowers consisting of 5 valves that have a shape of calyx with an intense red hue [1Sindi HA, Marshall LJ, Morgan MRA. Comparative chemical and biochemical analysis of extracts of Hibiscus sabdariffa. Food Chem 2014; 164: 23-9.
[http://dx.doi.org/10.1016/j.foodchem.2014.04.097] [PMID: 24996 300] ]; growing in tropical and subtropical areas of both hemispheres of the world [2Cisse M, Dornier M, Sakho M, Ndiaye A, Reynes M, Sock O. Le bissap (Hibiscus sabdariffa L.): composition et principales utilisations. Fruits 2009; 64: 179-93.
[http://dx.doi.org/10.1051/fruits/2009013] ].
Hibiscus calyx is widely used to make infusions or teas, as well as in the preparation of jellies and/or as a natural dye source [3Mahadevan N, Shivali KP, Kamboj P. Hibiscus sabdariffa Linn - an overview. Nat Prod Rad 2009; 8: 77-83.]. In traditional medicine, the hibiscus is used in the form of tea to treat several disorders such as constipation, cancer, heart disease, urinary tract infections, diabetes, high blood pressure, and hepatic disorders [4McKay DL, Chen CYO, Saltzman E, Blumberg JB. Hibiscus sabdariffa L. tea (tisane) lowers blood pressure in prehypertensive and mildly hypertensive adults. J Nutr 2010; 140(2): 298-303.
[http://dx.doi.org/10.3945/jn.109.115097] [PMID: 20018807] -6Fernández-Arroyo S, Rodríguez-Medina IC, Beltrán-Debón R, et al. Quantification of the polyphenolic fraction and in vitrol antioxidant and in vivo anti-hyperlipemic activities of Hibiscus sabdariffa aqueous extract. Food Res Int 2011; 44: 1490-5.
[http://dx.doi.org/10.1016/j.foodres.2011.03.040] ].
Nowadays, natural antioxidants from plant sources have had special importance, especially compounds, such as polyphenols and flavonoids (anthocyanins) [1Sindi HA, Marshall LJ, Morgan MRA. Comparative chemical and biochemical analysis of extracts of Hibiscus sabdariffa. Food Chem 2014; 164: 23-9.
[http://dx.doi.org/10.1016/j.foodchem.2014.04.097] [PMID: 24996 300] ]. Several studies have reported on the antioxidant capacity of hibiscus [7Ramakrishna BV, Jayaprakasha GK, Jena BS, Singh RP. Antioxidant activities of roselle (Hibiscus abdariffa) calyces and fruit extracts. J Food Sci Technol 2008; 45: 223-7.], as well as activity anti-inflammatory [8Fakeye TO, Pal A, Bawankule DU, Khanuja SPS. Immunomodulatory effect of extracts of Hibiscus sabdariffa L. (Family Malvaceae) in a mouse model. Phytother Res 2008; 22(5): 664-8.
[http://dx.doi.org/10.1002/ptr.2370] [PMID: 18398929] ], antimicrobial [9Jaroni D, Ravishankar S. Bactericidal effects of roselle (Hibiscus sabdariffa) against foodborne pathogens in vitrol and on romaine lettuce and alfalfa sprouts. Qual Assur Saf Crops Foods 2012; 4: 33-40.
[http://dx.doi.org/10.1111/j.1757-837X.2011.00117.x] ], anticholesterol [10Lin TL, Lin HH, Chen CC, Lin MC, Chou MC, Wang CJ. Hibiscus sabdariffa extract reduces serum cholesterol in men and women. Nutr Res 2007; 27: 140-5.
[http://dx.doi.org/10.1016/j.nutres.2007.01.007] ], hepatoprotective [11Yin G, Cao L, Xu P, Jeney G, Nakao M. Hepatoprotective and antioxidant effects of Hibiscus sabdariffa extract against carbon tetrachloride-induced hepatocyte damage in Cyprinus carpio. in vitro Cell Dev Biol Anim 2011; 47(1): 10-5.
[http://dx.doi.org/10.1007/s11626-010-9359-2] [PMID: 21082285] ], anticancer [12Lin HH, Chen JH, Kuo WH, Wang CJ. Chemopreventive properties of Hibiscus sabdariffa L. on human gastric carcinoma cells through apoptosis induction and JNK/p38 MAPK signaling activation. Chem Biol Interact 2007; 165(1): 59-75.
[http://dx.doi.org/10.1016/j.cbi.2006.10.011] [PMID: 17145051] ],
antihypertensive [13Herrera-Arellano A, Miranda-Sánchez J, Ávila-Castro P, et al. Clinical effects produced by a standardized herbal medicinal product of Hibiscus sabdariffa on patients with hypertension. A randomized, double-blind, lisinopril-controlled clinical trial. Planta Med 2007; 73(1): 6-12.
[http://dx.doi.org/10.1055/s-2006-957065] [PMID: 17315307] ], cardioprotective [14Alarcón-Alonso J, Zamilpa A, Aguilar FA, Herrera-Ruiz M, Tortoriello J, Jimenez-Ferrer E. Pharmacological characterization of the diuretic effect of Hibiscus sabdariffa Linn (Malvaceae) extract. J Ethnopharmacol 2012; 139(3): 751-6.
[http://dx.doi.org/10.1016/j.jep.2011.12.005] [PMID: 22178178] , 5Fernández-Arroyo S, Herranz-López M, Beltrán-Debón R, et al. Bioavailability study of a polyphenol-enriched extract from Hibiscus sabdariffa in rats and associated antioxidant status. Mol Nutr Food Res 2012; 56(10): 1590-5.
[http://dx.doi.org/10.1002/mnfr.201200091] [PMID: 22893520] ], anti-adipogenic [15Herranz-López M, Fernández-Arroyo S, Pérez-Sanchez A, et al. Synergism of plant-derived polyphenols in adipogenesis: perspectives and implications. Phytomedicine 2012; 19(3-4): 253-61.
[http://dx.doi.org/10.1016/j.phymed.2011.12.001] [PMID: 22280831] ], immunomodulatory [8Fakeye TO, Pal A, Bawankule DU, Khanuja SPS. Immunomodulatory effect of extracts of Hibiscus sabdariffa L. (Family Malvaceae) in a mouse model. Phytother Res 2008; 22(5): 664-8.
[http://dx.doi.org/10.1002/ptr.2370] [PMID: 18398929] ], and diuretic activity [14Alarcón-Alonso J, Zamilpa A, Aguilar FA, Herrera-Ruiz M, Tortoriello J, Jimenez-Ferrer E. Pharmacological characterization of the diuretic effect of Hibiscus sabdariffa Linn (Malvaceae) extract. J Ethnopharmacol 2012; 139(3): 751-6.
[http://dx.doi.org/10.1016/j.jep.2011.12.005] [PMID: 22178178] ]. The compounds responsible for these effects are the molecules of phenolic and anthocyanins present in hibiscus [6Fernández-Arroyo S, Rodríguez-Medina IC, Beltrán-Debón R, et al. Quantification of the polyphenolic fraction and in vitrol antioxidant and in vivo anti-hyperlipemic activities of Hibiscus sabdariffa aqueous extract. Food Res Int 2011; 44: 1490-5.
[http://dx.doi.org/10.1016/j.foodres.2011.03.040] , 16Prenesti E, Berto S, Daniele PG, Toso S. Antioxidant power quantification of decoction and cold infusions of Hibiscus sabdariffa flowers. Food Chem 2007; 100: 433-8.
[http://dx.doi.org/10.1016/j.foodchem.2005.09.063] ].
The extraction of bioactive compounds from natural sources has become important due to their use as phytochemicals in the preparation of functional food ingredients, food supplements, and additives, as well as pharmaceutical and cosmetic products [17Cisse M, Bohuon P, Sambe F, Kane C, Sakho M, Dornier M. Aqueous extraction of anthocyanins from Hibiscus sabdariffa: Experimental kinetics and modeling. J Food Eng 2012; 109: 16-21.
[http://dx.doi.org/10.1016/j.jfoodeng.2011.10.012] ]. However, the extraction of bioactive compounds from the fruits and vegetable sources depends on several factors, such as the solvent type, stirring speed, solid and solvent ratio, extraction time and temperature [18Luthria DL, Mukhopadhyay S, Kwansa AL. A systematic approach for extraction of phenolic compounds using parsley (Petroselinum crispum) flakes as a model substrate. J Sci Food Agric 2006; 86: 1350-8.
[http://dx.doi.org/10.1002/jsfa.2521] ]. In addition, several works have reported the use of mixed of enzymes such as pectinases, cellulases, and proteases, in order to increase the yield of the extraction of bioactive compounds in foods [19Nagendra chari KL, Manasa D, Srinivas P, Sowbhagya HB. Enzyme-assisted extraction of bioactive compounds from ginger (Zingiber officinale Roscoe). Food Chem 2013; 139(1-4): 509-14.
[http://dx.doi.org/10.1016/j.foodchem.2013.01.099] [PMID: 23561 138] , 20Benucci I, Río Segade S, Cerreti M, et al. Application of enzyme preparations for extraction of berry skin phenolics in withered winegrapes. Food Chem 2017; 237: 756-65.
[http://dx.doi.org/10.1016/j.foodchem.2017.06.003] [PMID: 28764 064] ].
The anthocyanins are commonly used in the food industry due to their coloring properties, which can provide several hues and chromas in food [21Reyes LF, Cisneros-Zevallos L. Degradation kinetics and colour of anthocyanins in aqueous extracts of purple and red-flesh potatoes (Solanum tuberosum L.). Food Chem 2007; 100: 885-94.
[http://dx.doi.org/10.1016/j.foodchem.2005.11.002] ]. Many edible plants, including the hibiscus calyces, are sources of anthocyanins, representing the largest group of water-soluble pigments in the plant [2Cisse M, Dornier M, Sakho M, Ndiaye A, Reynes M, Sock O. Le bissap (Hibiscus sabdariffa L.): composition et principales utilisations. Fruits 2009; 64: 179-93.
[http://dx.doi.org/10.1051/fruits/2009013] , 22Wong PK, Yusof S, Ghazali HM, Man YBC. Physico-chemical characteristics of roselle (Hibiscus sabdariffa L.). Nutr Food Sci 2002; 32: 68-73.
[http://dx.doi.org/10.1108/00346650210416994] ]. According to Da-Costa-Rocha et al. [23Da-Costa-Rocha I, Bonnlaender B, Sievers H, Pischel I, Heinrich M. Hibiscus sabdariffa L. - a phytochemical and pharmacological review. Food Chem 2014; 165: 424-43.
[http://dx.doi.org/10.1016/j.foodchem.2014.05.002] [PMID: 25038 696] ], aqueous extracts from the calyces of hibiscus contain two main anthocyanins: cyanidin 3-sambubioside and delphinidin 3-sambubioside, besides other phenolic compounds as phenolic acids.
In this context, the aim of this work was the extraction and quantification of the major phenolic compounds from hibiscus calyces, obtained by different conditions, using acidulated water as a solvent.
2. MATERIAL AND METHODS
2.1. Raw Material
The hibiscus was supplied for Association Lomba do Pinheiro, Brazil. Once received in the laboratory, the calyces were separated from the seeds. Then, calyces were cleaned, dried and packed in polyethylene bags, sealed and storage at -18 °C.
2.2. Chemicals
Enzyme complex of cellulose, hemicellulase and pectinase (Novozym 33095) was donated by Novozymes (Spain). HPLC-grade methanol was supplied by J.T. Baker (Trinidad and Tobago), formic acid and acetonitrile from Panreac (Darmstadt, Germany). Millipore (Massachusetts, USA) membranes (0.22 μm and 0.45 μm) were used before the HPLC analyses. The ABTS (2,2’azino-bis-3-ethylbenzo-thiazoline-6-sulfonic acid), trolox (6-hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid), standards caffeic acid, coumaric acid, ferulic acid, quercetin and cyanidin were purchased from Sigma-Aldrich (St. Louis, USA). All reagents were analytical grade.
2.3. Aqueous Extraction
The calyces were submitted to steam blanching for 4 min by autoclave at 100 ºC and right away, cooled in an ice bath for 3 min. The acidified water (2% of citric acid, w/v) [24Cid-Ortega S, Guerrero-Beltrán JA. Antioxidant and physicochemical properties of Hibiscus Sabdariffa extracts from two particle sizes. J Food Res 2016; 5: 98-109.
[http://dx.doi.org/10.5539/jfr.v5n2p98] ] was added to the calyces in a proportion of 1:5 (w/w), and the mixture was mixed and triturated in a blender (Britânia).
During the extraction procedure, the agitation was realized in a mechanical stirrer (RW20, IKA) with a rod stirrer (R1342). The temperature was controlled by a water bath (Laborota 4000, Heidoplph). After the extraction process, the extracts were centrifuged at 6.000 rpm (CR21GIII, Hitachi Koki) to separate the water-insoluble solids.
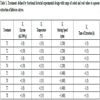
Color parameters of the hibiscus calyces extracts prepared following the treatments defined by fractional factorial experimental design showed in (Table 1).
2.4. Colorimetric Analysis
The measured color of the extracts was made using a colorimeter (CR400/410, Minolta), according to the CIELAB (L*, a*, b*) system. Before measurement, the instrument was calibrated using a white ceramic plate. Chroma (C* = [a*2 + b*2]1/2) was calculated, which indicates color’s purity or saturation.
2.5. Total Monomeric Anthocyanins (TMA)
The total anthocyanin content was determined by the method of differential pH [25Lee J, Durst RW, Wrolstad RE. Determination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines by the pH differential method: collaborative study. J AOAC Int 2005; 88(5): 1269-78.
[PMID: 16385975] ]. The extracts were mixed with buffer solutions pH 1.0 and 4.5. The absorbance was measured in a spectrophotometer (Genesys S10, Thermo Scientific) at 520 and 700 nm. The concentrations were expressed in mg of delphinidin 3-sambubioside per g extract on a dry basis, using molecular weight 577 g/mol, and molar absorptivity 26000 L/mol/cm [26Cisse M, Vaillant F, Acosta O, Dhuique-Mayer C, Dornier M. Thermal degradation kinetics of anthocyanins from blood orange, blackberry, and roselle using the arrhenius, eyring, and ball models. J Agric Food Chem 2009; 57(14): 6285-91.
[http://dx.doi.org/10.1021/jf900836b] [PMID: 19545116] ].
2.6. Antioxidant Capacity (ABTS)
The radical scavenging ability of the extracts was measured by the ABTS (2,2’azino-bis-3-ethylbenzo-thiazoline-6-sulfonic acid) antioxidant capacity assay following the methodology recommended by Re et al. [27Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med 1999; 26(9-10): 1231-7.
[http://dx.doi.org/10.1016/S0891-5849(98)00315-3] [PMID: 10381 194] ]. The results were calculated based on a calibration curve of Trolox (1.5 – 20 µM) (R2 = 0.9995), and Trolox-equivalent antioxidant capacity (TEAC) values were expressed as µmol of Trolox per g extract on a dry basis.
2.7. Quantification of Phenolic Compounds
Determination of the phenolic compounds was realized according to Rodrigues et al. [28Rodrigues E, Mariutti LRB, Mercadante AZ. Carotenoids and phenolic compounds from Solanum sessiliflorum, an unexploited Amazonian fruit, and their scavenging capacities against reactive oxygen and nitrogen species. J Agric Food Chem 2013; 61(12): 3022-9.
[http://dx.doi.org/10.1021/jf3054214] [PMID: 23432472] ]. The liquid chromatographic analysis was carried out in a Shimadzu HPLC system with a diode array detector, using an Atlantis T3-RP C18 column (250 × 4.6 mm, 5 μm). In order to improve the peak of separation a Synergi Hydro-RP C18 column (250× 4.6 mm, 4 μm) was also used. Mobile phase consisting of two solvents (A) water acidified with formic acid (0.5%, v/v) and (B) acetonitrile acidified with formic acid (0.5%, v/v), and the following gradient was used: A/B from 99:1 to 50:50 (0 - 50 min), from 50:50 to 1:99 (50 - 55 min) and finally held for 5 min. Column conditions were of 29 °C, 0.7 mL/min and 20 μL for temperature, the flow rate of mobile phase and injection volume, respectively. The UV-vis spectra were monitored from 200 to 800 nm and the chromatograms were obtained at 260, 320, 360 and 520 nm [29Dal Magro L, Dalagnol LMG, Manfroi V, Hertz PF, Klein MP, Rodrigues RC. Synergistic effects of pectinex ultra Clear and Lallzyme Beta on yield and bioactive compounds extraction of Concord grape juice. Lebensm Wiss Technol 2016; 72: 157-65.
[http://dx.doi.org/10.1016/j.lwt.2016.04.046] ].
HPLC was connected in series to a diode array detector and a mass spectrometer with a Q-TOF analyzer and electrospray ionization source (micrOTOF-QIII, Bruker Daltonics). Electrospray ionization and MS analysis conditions were: capillary voltage 2.0kV (positive mode) and 3.0kV (negative mode), nitrogen was used as both the nebulizing gas (flow rate of 8 mL/min) and dry gas (pressure of 2 bar), probe temperature of 310 °C and the mass spectra scan from 100 to 700 m/z. MS2 was set in automatic mode applying fragmentation energy of 34 V.
The phenolic compounds were identified based on retention time and elution order in the reversed phase column, UV−Vis and MS spectrum characteristic compared to standards analyzed on similar conditions and data of the literature [30Piovesana A, Rodrigues E, Noreña CPZ. Composition analysis of carotenoids and phenolic compounds and antioxidant activity from hibiscus calyces (Hibiscus sabdariffa L.) by HPLC DAD MS/MS. Phytochemical Analysis 2018 https://onlinelibrary.wiley.com /doi/abs/10.1002/pca.2806].
The phenolic compounds were quantified by HPLC-DAD, using analytical curves with nine-point of caffeoylquinic acid, ferulic acid, quercetin and cyanidin. Data analysis was very well fixed to linear models (r2 > 0.99).
2.8. Experimental Design and Statistical Analysis
For this study, a 24-1 fractional factorial design was utilized (Table 1), resulting in eight treatments. Four independent factors at two levels were studied: enzyme (x1) (0; 50 µL/1000 g extract), extraction temperature (x2) (35; 55 °C), stirring speed (x3) (200; 400 rpm) and extraction time (x4) (3; 5 h).
The variables xi were coded as Xi, according to the equation:
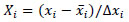 |
(1) |
Where 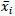 is the mean value of each independent variable, and ∆xi is the step change value. The levels of the independent variables in coded (-1, +1) and real values are shown in Table 1.
is the mean value of each independent variable, and ∆xi is the step change value. The levels of the independent variables in coded (-1, +1) and real values are shown in Table 1.
The following model was fitted to the data (Equation 1) through regression analysis:
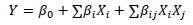 |
(2) |
Where Y is the response variable, β0, βi, and βij are coefficients of the term independent, the linear and interaction effects, respectively, and Xi and Xj the coded level of variables xi and xj.
The analysis of Chroma, TMA (total monomeric anthocyanins), ABTS and phenolic compounds were the responses variables. The resulting models were used to plot response surfaces. Data were subjected to ANOVA and Pearson correlation, and the treatments to Tukey’s multiple comparison tests, using the software SAS 9.3. The experiments were performed in triplicates and a 95% confidence level was used.
3. RESULTS AND DISCUSSION
3.1. Colorimetric Analysis
Table 2 shows the color parameters for different extraction conditions. L* values were significantly lower (darker color) when either higher levels of temperature or stirring speed were used. This might be due to the formation of dark compounds during the extraction. The higher values of a*, b* and Chroma were obtained at 35 ºC. The results also showed that they are located in the first quadrant of the hue circle, indicating a tendency to yellowness and redness. The best extracts with red color may be related to the extraction of anthocyanins [31Ramirez-Rodrigues MM, Plaza ML, Azeredo A, Balaban MO, Marshall MR. Physicochemical and phytochemical properties of cold and hot water extraction from Hibiscus sabdariffa. J Food Sci 2011; 76(3): C428-35.
[http://dx.doi.org/10.1111/j.1750-3841.2011.02091.x] [PMID: 21535 810] ].
3.2. Total Monomeric Anthocyanin (TMA)
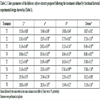
The mean values of TMA varied from 3.07 to 3.82 mg/g extract on a dry basis for the hibiscus calyces extracts Fig. (1A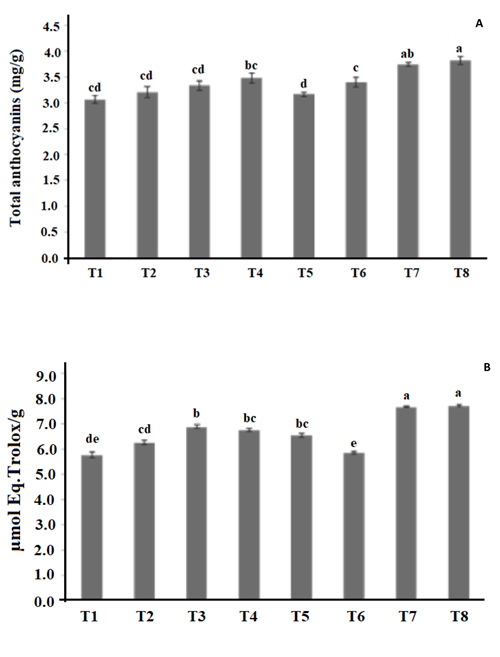 ), where the increase in the stirring speed and the temperature increased the TMA concentrations. These values are in agreement with Christian and Jackson [32Christian KR, Jackson JC. Changes in total phenolic and monomeric anthocyanin composition and antioxidant activity of three varieties of sorrel (Hibiscus sabdariffa) during maturity. J Food Compos Anal 2009; 22: 663-7.
), where the increase in the stirring speed and the temperature increased the TMA concentrations. These values are in agreement with Christian and Jackson [32Christian KR, Jackson JC. Changes in total phenolic and monomeric anthocyanin composition and antioxidant activity of three varieties of sorrel (Hibiscus sabdariffa) during maturity. J Food Compos Anal 2009; 22: 663-7.
[http://dx.doi.org/10.1016/j.jfca.2009.05.007] ] that reached mean values from 1.8 to 3.5 mg/g extract on a dry basis for TMA using organic solvents in the extraction. Thus, when used to ideal conditions in the hibiscus aqueous extraction process, it is possible to achieve similar levels of total monomeric anthocyanins compared with extraction with organic solvents. The highest anthocyanins extractions were obtained for T8 and T7 and they did not show a significant difference Fig. (1A ).
).
 |
Fig. (1) Determination of Total Monomeric Anthocyanins (TMA) (A) and ABTS (B) for the hibiscus calyces extracts prepared following the fractional factorial experimental design showed in Table 1. |
Phenolic compounds concentration in the hibiscus calyces extracts prepared following the fractional factorial experimental design showed in (Table 1).
When acid citric is added to extraction water, recovery of anthocyanins increase due to low pH of the solution denatures cell membranes permitting higher solubilization of the anthocyanins, and the free hydrogen ions allow stabilizing the red color produced by flavylium cation form of the anthocyanins [33Blackhall ML, Berry R, Davies NW, Walls JT. Optimized extraction of anthocyanins from Reid Fruits’ Prunus avium ‘Lapins’ cherries. Food Chem 2018; 256: 280-5.
[http://dx.doi.org/10.1016/j.foodchem.2018.02.137] [PMID: 29606 449] ]. In addition, at low pH, the flavylium is highly soluble in water [34Khoo HE, Azlan A, Tang ST, Lim SM. Anthocyanidins and anthocyanins: colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr Res 2017; 61(1): 1361779.
[http://dx.doi.org/10.1080/16546628.2017.1361779] [PMID: 28970 777] ].
3.3. Phenolic Compounds
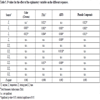
Fourteen phenolic compounds, namely, 3-caffeoylquinic acid, delphinidin 3-sambubioside, 3-p-coumaroylquinic acid, cyanidin 3-sambubioside, 5-caffeoylquinic acid, 4-caffeoylquinic acid, ferulic acid derived, myricetin 3-sambubioside, 5-p-coumaroylquinic acid, 5-O-caffeoylshikimic acid, quercetin 3-sambubioside, quercetin 3-rutinoside, quercetin 3-glucoside and kaempferol 3-O-rutinoside were identified [30Piovesana A, Rodrigues E, Noreña CPZ. Composition analysis of carotenoids and phenolic compounds and antioxidant activity from hibiscus calyces (Hibiscus sabdariffa L.) by HPLC DAD MS/MS. Phytochemical Analysis 2018 https://onlinelibrary.wiley.com /doi/abs/10.1002/pca.2806] and quantified. The concentrations on a dry basis of the phenolic compounds obtained by different extraction assays are shown in Table 3.
The total phenolic acids varied from 4165 to 6929 μg/g extract on dry basis, being the 3-caffeoylquinic acid the main phenolic acid of the hibiscus extract (2089 to 3568 μg/g extract on dry basis), followed, in descending order, by 5-caffeoylquinic acid (1726 to 2751 μg/g extract on dry basis), 3-p-Coumaroylquinic acid (163 to 310 μg/g extract on dry basis), 5-p-coumaroylquinic acid (72 to 127 μg/g extract on dry basis), 4-caffeoylquinic acid (63 to 107 μg/g extract on dry basis), 5-O-caffeoylshikimic acid (40 to 59 μg/g extract on dry basis) and ferulic acid derived (10 to 25 μg/g extract on dry basis). The T8 and T7 extractions provided the highest values of total phenolic acid (6929 and 6751 μg/g extract on dry basis, respectively), in these treatments can be observed that the use of both the higher levels of temperature and stirring speed, showing the importance of these variables for the extraction of phenolic acids. Several studies reported the importance of phenolic acid in antioxidant activity [35Borrás-Linares I, Fernández-Arroyo S, Arráez-Roman D, et al. Characterization of phenolic compounds, anthocyanidin, antioxidant and antimicrobial activity of 25 varieties of Mexican Roselle (Hibiscus sabdariffa). Ind Crops Prod 2015; 69: 385-94.
[http://dx.doi.org/10.1016/j.indcrop.2015.02.053] , 5Fernández-Arroyo S, Herranz-López M, Beltrán-Debón R, et al. Bioavailability study of a polyphenol-enriched extract from Hibiscus sabdariffa in rats and associated antioxidant status. Mol Nutr Food Res 2012; 56(10): 1590-5.
[http://dx.doi.org/10.1002/mnfr.201200091] [PMID: 22893520] ].
The content of total anthocyanins quantified by HPLC ranged from 5456 to 10084 μg/g extract on dry basis. The highest content for total anthocyanins was also obtained by T8 and T7 treatments, 10084 and 9395 μg/g extract on dry basis, respectively. These results were much higher than those found by Salazar-González et al. [36Salazar-González C, Vergara-Balderas FT, Ortega-Regules AE, Guerrero-Beltrán JÁ. Antioxidant properties and color of Hibiscus sabdariffa extracts. Cienc Investig Agrar 2012; 39: 79-90.
[http://dx.doi.org/10.4067/S0718-16202012000100006] ] who reported 2147 μg/g of total anthocyanins in hibiscus aqueous extract. Extraction efficiency depends on several variables, such as the technique of separation, the raw material composition, and the type of solvent [37Soquetta MB, Terra LM, Bastos CP. Green technologies for the extraction of bioactive compounds in fruits and vegetables. CYTA J Food 2018; 1: 400-12.
[http://dx.doi.org/10.1080/19476337.2017.1411978] ]. Thus, phenolic compounds profile contained in raw materials, for having different polarities, may affect the extraction process [38Andrade RAMS, Maciel MIS, Santos AMP, Melo EA. Optimization of the extraction process of polyphenols from cashew apple agro-industrial residues. Food Sci Technol 2015; 35: 354-60.
[http://dx.doi.org/10.1590/1678-457X.6585] ].
Regarding the anthocyanins of hibiscus extract, two anthocyanins, highlighting the delphinidin 3-sambubioside (up to 7516 μg/g extract on dry basis) as the main anthocyanin of the hibiscus were identified and quantified, followed by cyanidin-3-sambubioside (up to 2568 μg/g extract on dry basis), which is found to be in agreement with the results obtained by Ramirez-Rodrigues et al. [31Ramirez-Rodrigues MM, Plaza ML, Azeredo A, Balaban MO, Marshall MR. Physicochemical and phytochemical properties of cold and hot water extraction from Hibiscus sabdariffa. J Food Sci 2011; 76(3): C428-35.
[http://dx.doi.org/10.1111/j.1750-3841.2011.02091.x] [PMID: 21535 810] ] and Christian et al. [39Christian KR, Nair MG, Jackson JC. Antioxidant and cyclooxygenase inhibitory activity of sorrel (Hibiscus sabdariffa). J Food Compos Anal 2006; 19: 778-83.
[http://dx.doi.org/10.1016/j.jfca.2006.04.004] ], who reported delphinidin and cyanidin sambubiosides as the anthocyanins in hibiscus aqueous extracts.
Anthocyanins are responsible for the blue, red, purple and their complementary colors of most fruits, vegetables, and flowers [40Flores FP, Singh RK, Kong F. Anthocyanin extraction, microencapsulation, and release properties during in vitrol digestion. Food Rev Int 2016; 32: 46-67.
[http://dx.doi.org/10.1080/87559129.2015.1041185] ]. They constitute a large percentage of phenolic compounds from hibiscus, representing the most important compounds of the phenolic profile in this flower. These molecules have polar characteristic and are very reactive and water soluble [41Ongkowijoyo P, Luna-Vital DA, Gonzalez de Mejia E. Extraction techniques and analysis of anthocyanins from food sources by mass spectrometry: An update. Food Chem 2018; 250: 113-26.
[http://dx.doi.org/10.1016/j.foodchem.2018.01.055] [PMID: 29412 900] ], and for this reason, the water might be used as a solvent for extraction. Moreover, add a small amount of acid would allow rising the stability of them [41Ongkowijoyo P, Luna-Vital DA, Gonzalez de Mejia E. Extraction techniques and analysis of anthocyanins from food sources by mass spectrometry: An update. Food Chem 2018; 250: 113-26.
[http://dx.doi.org/10.1016/j.foodchem.2018.01.055] [PMID: 29412 900] ]. Besides, they are unstable at different factors, such as temperature, light, oxygen, pH, water activity and attendance of enzymes and copigments [42Chung C, Rojanasasithara T, Mutilangi W, McClements DJ. Stability improvement of natural food colors: Impact of amino acid and peptide addition on anthocyanin stability in model beverages. Food Chem 2017; 218: 277-84.
[http://dx.doi.org/10.1016/j.foodchem.2016.09.087] [PMID: 27719 910] , 43Weber F, Boch K, Schieber A. Influence of copigmentation on the stability of spray dried anthocyanins from blackberry. Lebensm Wiss Technol 2017; 75: 72-7.
[http://dx.doi.org/10.1016/j.lwt.2016.08.042] ].
For total flavonoids found in the hibiscus extracts, the values ranged from 349 to 598 μg/g extract on dry basis. The highest concentrations were achieved by T3 and T8 (518 and 581 μg/g extract on dry basis, respectively) while the lowest concentrations were obtained by T1 (349 μg/g extract on dry basis). Among the main flavonoids identified and quantified on the hibiscus calyces extracts, it can be highlighted the derivatives of quercetin, kaempferol, and myricetin, in accordance with the results reported by Borrás-Linares et al. [44Borrás-Linares I, Herranz-López M, Barrajón-Catalán E, et al. Permeability study of polyphenols derived from a phenolic-enriched Hibiscus sabdariffa extract by UHPLC-ESI-UHR-Qq-TOF-MS. Int J Mol Sci 2015; 16(8): 18396-411. b
[http://dx.doi.org/10.3390/ijms160818396] [PMID: 26262611] ].
In general, T8 provided the highest extraction of phenolic compounds (17595 μg/g extract on dry basis), followed by T7 (16656 μg/g extract on dry basis), showing a positive effect mainly of the temperature and stirring speed in the extraction of phenolic compounds. According to Pinelo et al. [45Pinelo M, Arnous A, Meyer AS. Upgrading of grape skins: significance of plant cell wall structural components and extraction techniques for phenol release. Trends Food Sci Technol 2006; 17: 579-90.
[http://dx.doi.org/10.1016/j.tifs.2006.05.003] ], the phenolic compounds can be linked or entangled in the polysaccharides of the cell walls, the cell vacuoles, or associated with cell nuclei.
Thus, it can be concluded that treatments in which high temperatures combined with high stirring speed provided greater degradation of hibiscus cells, increasing the release of intracellular components and of phenolic compounds bound to the cell wall polysaccharides. These results are in accordance with other studies that also emphasized the importance among temperature, time and stirring on the extraction of phenolic compounds [18Luthria DL, Mukhopadhyay S, Kwansa AL. A systematic approach for extraction of phenolic compounds using parsley (Petroselinum crispum) flakes as a model substrate. J Sci Food Agric 2006; 86: 1350-8.
[http://dx.doi.org/10.1002/jsfa.2521] , 45Pinelo M, Arnous A, Meyer AS. Upgrading of grape skins: significance of plant cell wall structural components and extraction techniques for phenol release. Trends Food Sci Technol 2006; 17: 579-90.
[http://dx.doi.org/10.1016/j.tifs.2006.05.003] ].
The extraction yields are shown in Table 3. The yields ranged from 28.36 to 52.42 and from 29.38 to 51.84 for anthocyanins and total phenolic compounds, respectively. The highest yield for these two compounds was obtained by T8 treatment.
3.4. Antioxidant Capacity ABTS
The highest antioxidant capacities measured by ABTS were obtained by T7 and T8 Fig. (1B ). These results are also in agreement with Wong et al. [46Wong PK, Yusof S, Ghazali HM, Man YBC. Optimization of hot water extraction of roselle juice using response surface methodology: a comparative study with other extraction methods. J Sci Food Agric 2003; 83: 1273-8.
). These results are also in agreement with Wong et al. [46Wong PK, Yusof S, Ghazali HM, Man YBC. Optimization of hot water extraction of roselle juice using response surface methodology: a comparative study with other extraction methods. J Sci Food Agric 2003; 83: 1273-8.
[http://dx.doi.org/10.1002/jsfa.1416] ], who observed that the antioxidant capacity values increased with the increase of temperature, due to the higher mass transfer and the higher separation of antioxidant phenolic compounds.
Thus, the highest antioxidant capacities were obtained by treatments that provided the highest extraction of phenolic compounds. It is important to highlight that several studies have correlated the antioxidant activity with the phenolic content [16Prenesti E, Berto S, Daniele PG, Toso S. Antioxidant power quantification of decoction and cold infusions of Hibiscus sabdariffa flowers. Food Chem 2007; 100: 433-8.
[http://dx.doi.org/10.1016/j.foodchem.2005.09.063] , 47Sánchez-Mendoza J, Domínguez-López A, Navarro-Galindo S, López-Sandoval JA. Some physical properties of Roselle (Hibiscus sabdariffa L.) seeds as a function of moisture content. J Food Eng 2008; 87: 391-7.
[http://dx.doi.org/10.1016/j.jfoodeng.2007.12.023] ].
Table 4 shows the Pearson correlation coefficients between the color parameters, phenolic acids, total phenolics, anthocyanins, total monomeric, flavonoids and ABTS in several extractions. The highest correlation coefficients were observed between ABTS (0.90) with phenolic acids, total phenolics, and anthocyanins. On the other hand, both parameters a* or b* were also highly correlated, but showed the negative relationship (ranged between -0.98 and -0.85 and -0.95 and 0.84, respectively) with phenolic acids, total phenolics, anthocyanins total monomeric, flavonoids and ABTS.
3.5. Effect of the Variables on the Responses
The equations that describe the behavior in the extraction of the Chroma (Y1), TMA (Y2), ABTS (Y3) and phenolic compounds (Y4) in relation to the responses variables are presented in the equations below.
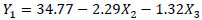 |
(3) |
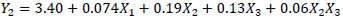 |
(4) |
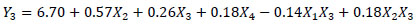 |
(5) |
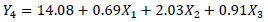 |
(6) |
Where, X1, X2, X3, and X4 are the coded independent variables for enzyme concentration, temperature, stirring speed and time, respectively. The significance of each coefficient and the coefficients of determination, the linear and interaction effects of the variables are shown in Table 5. For better understanding, contour lines plots for Chroma, TMA, ABTS and phenolic compounds were generated in accordance with Equations 3 to 6.
For Chroma only the linear effects of the temperature and stirring speed were significant (Equation 3 and Fig. 2A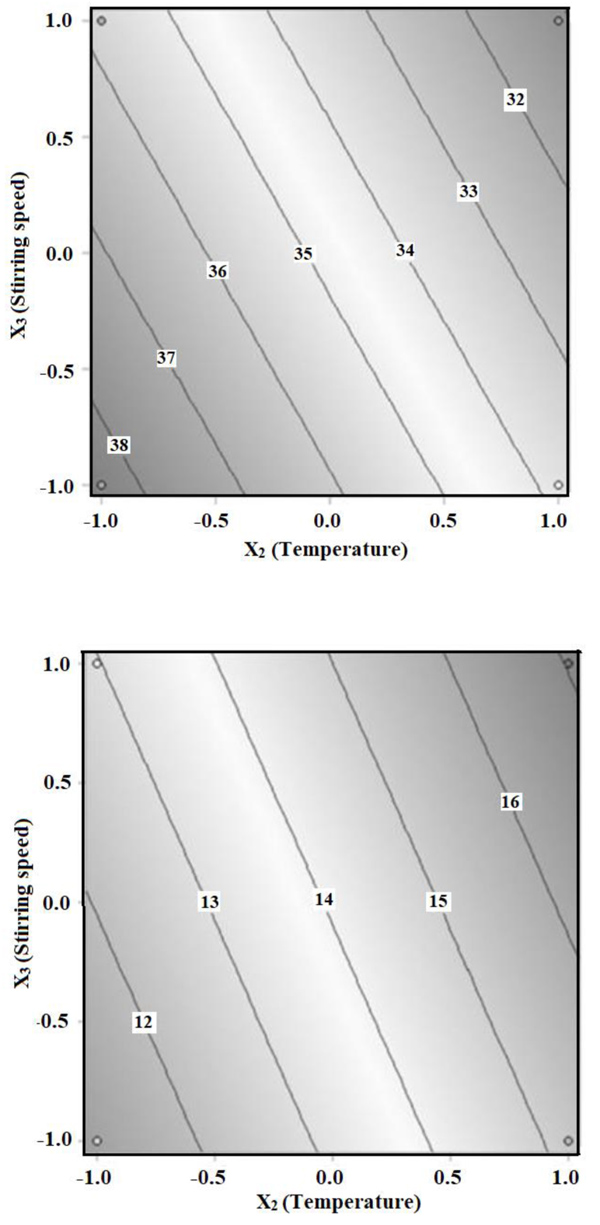 ). For phenolic compounds, the linear effects of the enzyme, temperature and stirring speed were significant (Equation 6 and Fig. 2B
). For phenolic compounds, the linear effects of the enzyme, temperature and stirring speed were significant (Equation 6 and Fig. 2B ). Three linear effects were significant for TMA (X1, X2 and X3) and ABTS (X2, X3 and X4). Furthermore, the interaction effect of X2X3 was significant for TMA, while X1X3 and X2X3 were significant for ABTS (Equations 4 and 5, Figs. (3A
). Three linear effects were significant for TMA (X1, X2 and X3) and ABTS (X2, X3 and X4). Furthermore, the interaction effect of X2X3 was significant for TMA, while X1X3 and X2X3 were significant for ABTS (Equations 4 and 5, Figs. (3A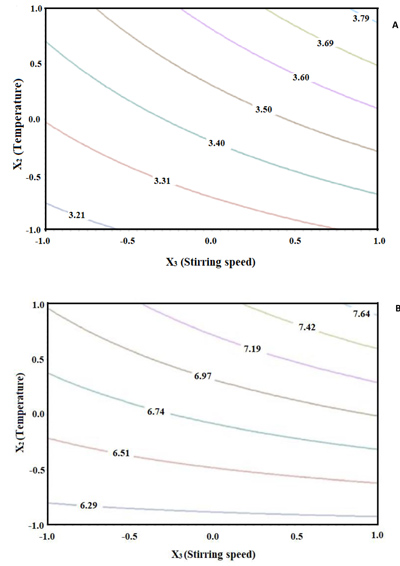 and 3B
and 3B ), respectively).
), respectively).
Chroma values increased when temperature and stirring speed decreased Fig. (2A ). On the other hand, in (Fig. 2B
). On the other hand, in (Fig. 2B ), it was observed that phenolic content increased with stirring speed and temperature. In (Figs. 3A
), it was observed that phenolic content increased with stirring speed and temperature. In (Figs. 3A and 3B
and 3B ), for anthocyanins (TMA) and ABTS can be observed a typical behavior of the interaction between factors, where the better extraction conditions were achieved when for these two variables (stirring speed and temperature) were used in their higher levels.
), for anthocyanins (TMA) and ABTS can be observed a typical behavior of the interaction between factors, where the better extraction conditions were achieved when for these two variables (stirring speed and temperature) were used in their higher levels.
It was also observed that the increase in the values of concentration of enzymes will increase response variables TMA and phenolic content. The use of commercial enzymes with pectinase, cellulase and protease activities help in the degradation of cell walls, and this way may facilitate the extraction of phenolic and anthocyanin compounds [20Benucci I, Río Segade S, Cerreti M, et al. Application of enzyme preparations for extraction of berry skin phenolics in withered winegrapes. Food Chem 2017; 237: 756-65.
[http://dx.doi.org/10.1016/j.foodchem.2017.06.003] [PMID: 28764 064] , 29Dal Magro L, Dalagnol LMG, Manfroi V, Hertz PF, Klein MP, Rodrigues RC. Synergistic effects of pectinex ultra Clear and Lallzyme Beta on yield and bioactive compounds extraction of Concord grape juice. Lebensm Wiss Technol 2016; 72: 157-65.
[http://dx.doi.org/10.1016/j.lwt.2016.04.046] ].
All dependent variables were temperature-dependents. . The temperature had a significant effect on Chroma (Y1), TMA (Y2), ABTS (Y3), and phenolic compounds (Y4). In addition, for TMA (Y2), temperature also caused interaction with stirring speed. Temperature is a variable of great importance in the extraction of phenolic compounds, due to its influence on the viscosity, solubility, rates of mass transfer, and mass diffusion coefficient.
 |
Fig. (2) Contour plots for stirring speed versus temperature in the hibiscus calyces extraction for Chroma (Y1) (A); and phenolic compounds (Y4) (B), where the variable X1 (enzyme) was fixed in 0.5. |
CONCLUSION
In this work, it was observed that the treatments that used the higher levels of temperature and stirring speed were able to improve the extraction of phenolic compounds in the hibiscus. The best extract was considered the treatment eight, that obtained a aqueous extract with higher concentrations of total phenolic acids (6.93 mg/g extract on dry basis), total anthocyanins (10.08 mg/g extract on dry basis), total flavonoids (0.58 mg/g extract on dry basis) and total phenolic compound (17.59 mg/g extract on dry basis). The use of acidulated water as a solvent has shown promising effects when compared with other studies that use organic solvent extraction used in the food industry, for example, for production of microcapsules or natural colorant.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
HUMAN AND ANIMAL RIGHTS
No animals/humans were used for studies that are the basis of this research.
CONSENT FOR PUBLICATION
Not applicable.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest financial or otherwise.
ACKNOWLEDGMENTS
We thank the Capes, CNPq and FAPERGS for their financial support.
REFERENCES
| [1] | Sindi HA, Marshall LJ, Morgan MRA. Comparative chemical and biochemical analysis of extracts of Hibiscus sabdariffa. Food Chem 2014; 164: 23-9. [http://dx.doi.org/10.1016/j.foodchem.2014.04.097] [PMID: 24996 300] |
| [2] | Cisse M, Dornier M, Sakho M, Ndiaye A, Reynes M, Sock O. Le bissap (Hibiscus sabdariffa L.): composition et principales utilisations. Fruits 2009; 64: 179-93. [http://dx.doi.org/10.1051/fruits/2009013] |
| [3] | Mahadevan N, Shivali KP, Kamboj P. Hibiscus sabdariffa Linn - an overview. Nat Prod Rad 2009; 8: 77-83. |
| [4] | McKay DL, Chen CYO, Saltzman E, Blumberg JB. Hibiscus sabdariffa L. tea (tisane) lowers blood pressure in prehypertensive and mildly hypertensive adults. J Nutr 2010; 140(2): 298-303. [http://dx.doi.org/10.3945/jn.109.115097] [PMID: 20018807] |
| [5] | Fernández-Arroyo S, Herranz-López M, Beltrán-Debón R, et al. Bioavailability study of a polyphenol-enriched extract from Hibiscus sabdariffa in rats and associated antioxidant status. Mol Nutr Food Res 2012; 56(10): 1590-5. [http://dx.doi.org/10.1002/mnfr.201200091] [PMID: 22893520] |
| [6] | Fernández-Arroyo S, Rodríguez-Medina IC, Beltrán-Debón R, et al. Quantification of the polyphenolic fraction and in vitrol antioxidant and in vivo anti-hyperlipemic activities of Hibiscus sabdariffa aqueous extract. Food Res Int 2011; 44: 1490-5. [http://dx.doi.org/10.1016/j.foodres.2011.03.040] |
| [7] | Ramakrishna BV, Jayaprakasha GK, Jena BS, Singh RP. Antioxidant activities of roselle (Hibiscus abdariffa) calyces and fruit extracts. J Food Sci Technol 2008; 45: 223-7. |
| [8] | Fakeye TO, Pal A, Bawankule DU, Khanuja SPS. Immunomodulatory effect of extracts of Hibiscus sabdariffa L. (Family Malvaceae) in a mouse model. Phytother Res 2008; 22(5): 664-8. [http://dx.doi.org/10.1002/ptr.2370] [PMID: 18398929] |
| [9] | Jaroni D, Ravishankar S. Bactericidal effects of roselle (Hibiscus sabdariffa) against foodborne pathogens in vitrol and on romaine lettuce and alfalfa sprouts. Qual Assur Saf Crops Foods 2012; 4: 33-40. [http://dx.doi.org/10.1111/j.1757-837X.2011.00117.x] |
| [10] | Lin TL, Lin HH, Chen CC, Lin MC, Chou MC, Wang CJ. Hibiscus sabdariffa extract reduces serum cholesterol in men and women. Nutr Res 2007; 27: 140-5. [http://dx.doi.org/10.1016/j.nutres.2007.01.007] |
| [11] | Yin G, Cao L, Xu P, Jeney G, Nakao M. Hepatoprotective and antioxidant effects of Hibiscus sabdariffa extract against carbon tetrachloride-induced hepatocyte damage in Cyprinus carpio. in vitro Cell Dev Biol Anim 2011; 47(1): 10-5. [http://dx.doi.org/10.1007/s11626-010-9359-2] [PMID: 21082285] |
| [12] | Lin HH, Chen JH, Kuo WH, Wang CJ. Chemopreventive properties of Hibiscus sabdariffa L. on human gastric carcinoma cells through apoptosis induction and JNK/p38 MAPK signaling activation. Chem Biol Interact 2007; 165(1): 59-75. [http://dx.doi.org/10.1016/j.cbi.2006.10.011] [PMID: 17145051] |
| [13] | Herrera-Arellano A, Miranda-Sánchez J, Ávila-Castro P, et al. Clinical effects produced by a standardized herbal medicinal product of Hibiscus sabdariffa on patients with hypertension. A randomized, double-blind, lisinopril-controlled clinical trial. Planta Med 2007; 73(1): 6-12. [http://dx.doi.org/10.1055/s-2006-957065] [PMID: 17315307] |
| [14] | Alarcón-Alonso J, Zamilpa A, Aguilar FA, Herrera-Ruiz M, Tortoriello J, Jimenez-Ferrer E. Pharmacological characterization of the diuretic effect of Hibiscus sabdariffa Linn (Malvaceae) extract. J Ethnopharmacol 2012; 139(3): 751-6. [http://dx.doi.org/10.1016/j.jep.2011.12.005] [PMID: 22178178] |
| [15] | Herranz-López M, Fernández-Arroyo S, Pérez-Sanchez A, et al. Synergism of plant-derived polyphenols in adipogenesis: perspectives and implications. Phytomedicine 2012; 19(3-4): 253-61. [http://dx.doi.org/10.1016/j.phymed.2011.12.001] [PMID: 22280831] |
| [16] | Prenesti E, Berto S, Daniele PG, Toso S. Antioxidant power quantification of decoction and cold infusions of Hibiscus sabdariffa flowers. Food Chem 2007; 100: 433-8. [http://dx.doi.org/10.1016/j.foodchem.2005.09.063] |
| [17] | Cisse M, Bohuon P, Sambe F, Kane C, Sakho M, Dornier M. Aqueous extraction of anthocyanins from Hibiscus sabdariffa: Experimental kinetics and modeling. J Food Eng 2012; 109: 16-21. [http://dx.doi.org/10.1016/j.jfoodeng.2011.10.012] |
| [18] | Luthria DL, Mukhopadhyay S, Kwansa AL. A systematic approach for extraction of phenolic compounds using parsley (Petroselinum crispum) flakes as a model substrate. J Sci Food Agric 2006; 86: 1350-8. [http://dx.doi.org/10.1002/jsfa.2521] |
| [19] | Nagendra chari KL, Manasa D, Srinivas P, Sowbhagya HB. Enzyme-assisted extraction of bioactive compounds from ginger (Zingiber officinale Roscoe). Food Chem 2013; 139(1-4): 509-14. [http://dx.doi.org/10.1016/j.foodchem.2013.01.099] [PMID: 23561 138] |
| [20] | Benucci I, Río Segade S, Cerreti M, et al. Application of enzyme preparations for extraction of berry skin phenolics in withered winegrapes. Food Chem 2017; 237: 756-65. [http://dx.doi.org/10.1016/j.foodchem.2017.06.003] [PMID: 28764 064] |
| [21] | Reyes LF, Cisneros-Zevallos L. Degradation kinetics and colour of anthocyanins in aqueous extracts of purple and red-flesh potatoes (Solanum tuberosum L.). Food Chem 2007; 100: 885-94. [http://dx.doi.org/10.1016/j.foodchem.2005.11.002] |
| [22] | Wong PK, Yusof S, Ghazali HM, Man YBC. Physico-chemical characteristics of roselle (Hibiscus sabdariffa L.). Nutr Food Sci 2002; 32: 68-73. [http://dx.doi.org/10.1108/00346650210416994] |
| [23] | Da-Costa-Rocha I, Bonnlaender B, Sievers H, Pischel I, Heinrich M. Hibiscus sabdariffa L. - a phytochemical and pharmacological review. Food Chem 2014; 165: 424-43. [http://dx.doi.org/10.1016/j.foodchem.2014.05.002] [PMID: 25038 696] |
| [24] | Cid-Ortega S, Guerrero-Beltrán JA. Antioxidant and physicochemical properties of Hibiscus Sabdariffa extracts from two particle sizes. J Food Res 2016; 5: 98-109. [http://dx.doi.org/10.5539/jfr.v5n2p98] |
| [25] | Lee J, Durst RW, Wrolstad RE. Determination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines by the pH differential method: collaborative study. J AOAC Int 2005; 88(5): 1269-78. [PMID: 16385975] |
| [26] | Cisse M, Vaillant F, Acosta O, Dhuique-Mayer C, Dornier M. Thermal degradation kinetics of anthocyanins from blood orange, blackberry, and roselle using the arrhenius, eyring, and ball models. J Agric Food Chem 2009; 57(14): 6285-91. [http://dx.doi.org/10.1021/jf900836b] [PMID: 19545116] |
| [27] | Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med 1999; 26(9-10): 1231-7. [http://dx.doi.org/10.1016/S0891-5849(98)00315-3] [PMID: 10381 194] |
| [28] | Rodrigues E, Mariutti LRB, Mercadante AZ. Carotenoids and phenolic compounds from Solanum sessiliflorum, an unexploited Amazonian fruit, and their scavenging capacities against reactive oxygen and nitrogen species. J Agric Food Chem 2013; 61(12): 3022-9. [http://dx.doi.org/10.1021/jf3054214] [PMID: 23432472] |
| [29] | Dal Magro L, Dalagnol LMG, Manfroi V, Hertz PF, Klein MP, Rodrigues RC. Synergistic effects of pectinex ultra Clear and Lallzyme Beta on yield and bioactive compounds extraction of Concord grape juice. Lebensm Wiss Technol 2016; 72: 157-65. [http://dx.doi.org/10.1016/j.lwt.2016.04.046] |
| [30] | Piovesana A, Rodrigues E, Noreña CPZ. Composition analysis of carotenoids and phenolic compounds and antioxidant activity from hibiscus calyces (Hibiscus sabdariffa L.) by HPLC DAD MS/MS. Phytochemical Analysis 2018 https://onlinelibrary.wiley.com /doi/abs/10.1002/pca.2806 |
| [31] | Ramirez-Rodrigues MM, Plaza ML, Azeredo A, Balaban MO, Marshall MR. Physicochemical and phytochemical properties of cold and hot water extraction from Hibiscus sabdariffa. J Food Sci 2011; 76(3): C428-35. [http://dx.doi.org/10.1111/j.1750-3841.2011.02091.x] [PMID: 21535 810] |
| [32] | Christian KR, Jackson JC. Changes in total phenolic and monomeric anthocyanin composition and antioxidant activity of three varieties of sorrel (Hibiscus sabdariffa) during maturity. J Food Compos Anal 2009; 22: 663-7. [http://dx.doi.org/10.1016/j.jfca.2009.05.007] |
| [33] | Blackhall ML, Berry R, Davies NW, Walls JT. Optimized extraction of anthocyanins from Reid Fruits’ Prunus avium ‘Lapins’ cherries. Food Chem 2018; 256: 280-5. [http://dx.doi.org/10.1016/j.foodchem.2018.02.137] [PMID: 29606 449] |
| [34] | Khoo HE, Azlan A, Tang ST, Lim SM. Anthocyanidins and anthocyanins: colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr Res 2017; 61(1): 1361779. [http://dx.doi.org/10.1080/16546628.2017.1361779] [PMID: 28970 777] |
| [35] | Borrás-Linares I, Fernández-Arroyo S, Arráez-Roman D, et al. Characterization of phenolic compounds, anthocyanidin, antioxidant and antimicrobial activity of 25 varieties of Mexican Roselle (Hibiscus sabdariffa). Ind Crops Prod 2015; 69: 385-94. [http://dx.doi.org/10.1016/j.indcrop.2015.02.053] |
| [36] | Salazar-González C, Vergara-Balderas FT, Ortega-Regules AE, Guerrero-Beltrán JÁ. Antioxidant properties and color of Hibiscus sabdariffa extracts. Cienc Investig Agrar 2012; 39: 79-90. [http://dx.doi.org/10.4067/S0718-16202012000100006] |
| [37] | Soquetta MB, Terra LM, Bastos CP. Green technologies for the extraction of bioactive compounds in fruits and vegetables. CYTA J Food 2018; 1: 400-12. [http://dx.doi.org/10.1080/19476337.2017.1411978] |
| [38] | Andrade RAMS, Maciel MIS, Santos AMP, Melo EA. Optimization of the extraction process of polyphenols from cashew apple agro-industrial residues. Food Sci Technol 2015; 35: 354-60. [http://dx.doi.org/10.1590/1678-457X.6585] |
| [39] | Christian KR, Nair MG, Jackson JC. Antioxidant and cyclooxygenase inhibitory activity of sorrel (Hibiscus sabdariffa). J Food Compos Anal 2006; 19: 778-83. [http://dx.doi.org/10.1016/j.jfca.2006.04.004] |
| [40] | Flores FP, Singh RK, Kong F. Anthocyanin extraction, microencapsulation, and release properties during in vitrol digestion. Food Rev Int 2016; 32: 46-67. [http://dx.doi.org/10.1080/87559129.2015.1041185] |
| [41] | Ongkowijoyo P, Luna-Vital DA, Gonzalez de Mejia E. Extraction techniques and analysis of anthocyanins from food sources by mass spectrometry: An update. Food Chem 2018; 250: 113-26. [http://dx.doi.org/10.1016/j.foodchem.2018.01.055] [PMID: 29412 900] |
| [42] | Chung C, Rojanasasithara T, Mutilangi W, McClements DJ. Stability improvement of natural food colors: Impact of amino acid and peptide addition on anthocyanin stability in model beverages. Food Chem 2017; 218: 277-84. [http://dx.doi.org/10.1016/j.foodchem.2016.09.087] [PMID: 27719 910] |
| [43] | Weber F, Boch K, Schieber A. Influence of copigmentation on the stability of spray dried anthocyanins from blackberry. Lebensm Wiss Technol 2017; 75: 72-7. [http://dx.doi.org/10.1016/j.lwt.2016.08.042] |
| [44] | Borrás-Linares I, Herranz-López M, Barrajón-Catalán E, et al. Permeability study of polyphenols derived from a phenolic-enriched Hibiscus sabdariffa extract by UHPLC-ESI-UHR-Qq-TOF-MS. Int J Mol Sci 2015; 16(8): 18396-411. b [http://dx.doi.org/10.3390/ijms160818396] [PMID: 26262611] |
| [45] | Pinelo M, Arnous A, Meyer AS. Upgrading of grape skins: significance of plant cell wall structural components and extraction techniques for phenol release. Trends Food Sci Technol 2006; 17: 579-90. [http://dx.doi.org/10.1016/j.tifs.2006.05.003] |
| [46] | Wong PK, Yusof S, Ghazali HM, Man YBC. Optimization of hot water extraction of roselle juice using response surface methodology: a comparative study with other extraction methods. J Sci Food Agric 2003; 83: 1273-8. [http://dx.doi.org/10.1002/jsfa.1416] |
| [47] | Sánchez-Mendoza J, Domínguez-López A, Navarro-Galindo S, López-Sandoval JA. Some physical properties of Roselle (Hibiscus sabdariffa L.) seeds as a function of moisture content. J Food Eng 2008; 87: 391-7. [http://dx.doi.org/10.1016/j.jfoodeng.2007.12.023] |