- Home
- About Journals
-
Information for Authors/ReviewersEditorial Policies
Publication Fee
Publication Cycle - Process Flowchart
Online Manuscript Submission and Tracking System
Publishing Ethics and Rectitude
Authorship
Author Benefits
Reviewer Guidelines
Guest Editor Guidelines
Peer Review Workflow
Quick Track Option
Copyediting Services
Bentham Open Membership
Bentham Open Advisory Board
Archiving Policies
Fabricating and Stating False Information
Post Publication Discussions and Corrections
Editorial Management
Advertise With Us
Funding Agencies
Rate List
Kudos
General FAQs
Special Fee Waivers and Discounts
- Contact
- Help
- About Us
- Search

The Open Toxicology Journal
(Discontinued)
ISSN: 1874-3404 ― Volume 7, 2021
High Glucose Enhances Skin Sensitizer-induced NRF2 Activation In Vitro
Takeo Takeda1, Masahiro Ogawa1, *, Junya Kitamoto1, Takahiro Kyoya1, Megumi Terada1
Abstract
Background:
Hyperglycemia has a potentially critical role in the promotion of sensitization, however, the clear mechanism of this phenomenon is unknown. The activation of NRF2 is a key event triggered by skin sensitizers. Therefore, we investigated the effects of high glucose on the activation of NRF2 by the skin sensitizers in vitro.
Methods:
The involvement of glucose levels in NRF2 activation by cinnamaldehyde, a skin sensitizer, was assessed in human MCF-7 breast cancer cells under normal glucose conditions (1.0 g/L D-glucose) and high glucose conditions (4.5 g/L D-glucose).
Results:
High glucose induced the NRF2 transactivation, HMOX1 mRNA expression, and SOD-like activity. Nuclear NRF2 level was increased under high glucose conditions compared to normal glucose conditions. High glucose also enhanced the cinnamaldehyde-induced HMOX1 mRNA expression and SOD-like activity.
Conclusion:
Oxidative stress caused by hyperglycemia induced additionally the activation of NRF2 signaling by skin sensitizers.
Article Information
Identifiers and Pagination:
Year: 2021Volume: 7
First Page: 8
Last Page: 17
Publisher Id: TOTOXIJ-7-8
DOI: 10.2174/1874340402107010008
Article History:
Received Date: 22/10/2020Revision Received Date: 3/3/2021
Acceptance Date: 12/3/2021
Electronic publication date: 19/4/2021
Collection year: 2021
open-access license: This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International Public License (CC-BY 4.0), a copy of which is available at: https://creativecommons.org/licenses/by/4.0/legalcode. This license permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
* Address correspondence to this author at the Life Science Research Institute, Kumiai Chemical Industry Co. Ltd., Shizuoka, Japan; Tel: +81-537-35-3160; Fax: +81-537-36-3718; E-mail: m-ogawa@kumiai-chem.co.jp
| Open Peer Review Details | |||
|---|---|---|---|
| Manuscript submitted on 22-10-2020 |
Original Manuscript | High Glucose Enhances Skin Sensitizer-induced NRF2 Activation In Vitro | |
1. INTRODUCTION
Hyperglycemia, blood glucose levels above 2.0 g/L, is one of the major pathophysiological factors causing late complications in diabetes [1Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res 2010; 107(9): 1058-70.
[http://dx.doi.org/10.1161/CIRCRESAHA.110.223545] [PMID: 21030723] , 2Oguntibeju OO. Type 2 diabetes mellitus, oxidative stress and inflammation: Examining the links. Int J Physiol Pathophysiol Pharmacol 2019; 11(3): 45-63.
[PMID: 31333808] ]. Reactive Oxygen Species (ROS) are increased by hyperglycemia [3King GL, Loeken MR. Hyperglycemia-induced oxidative stress in diabetic complications. Histochem Cell Biol 2004; 122(4): 333-8.
[http://dx.doi.org/10.1007/s00418-004-0678-9] [PMID: 15257460] ]. Oxidative damage in individual cells may reach a sufficient threshold to cause DNA strand breaks and induce cell death [4Du X, Matsumura T, Edelstein D, et al. Inhibition of GAPDH activity by poly(ADP-ribose) polymerase activates three major pathways of hyperglycemic damage in endothelial cells. J Clin Invest 2003; 112(7): 1049-57.
[http://dx.doi.org/10.1172/JCI18127] [PMID: 14523042] ]. Therefore, hyperglycemia-induced oxidative stress is one of the responsible factors for the pathology of diabetic complications.
The frequency of allergic diseases, such as allergic rhinitis, asthma, and food allergy, has been increased dramatically over the past decade [5Bach JF. The effect of infections on susceptibility to autoimmune and allergic diseases. N Engl J Med 2002; 347(12): 911-20.
[http://dx.doi.org/10.1056/NEJMra020100] [PMID: 12239261] ]. Allergies are highly dependent on both environmental factors (allergens, tobacco smoke, and indoor and outdoor air pollution) and internal factors (hormones, diet, and circadian clock). Additionally, there is a high prevalence of allergic sensitization in Type 1 diabetic patients [6Tosca MA, Silvestri M, Olcese R, et al. Allergic sensitization and symptoms, body mass index, and respiratory function in children with type 1 diabetes mellitus. Ann Allergy Asthma Immunol 2012; 108(2): 128-9.
[http://dx.doi.org/10.1016/j.anai.2011.12.002] [PMID: 22289736] ]. A recent study has shown that hyperglycemia promotes sensitization in a mouse model for asthma [7Queener A, Jeong BM, Doan TC, et al. Induced hyperglycemia promotes sensitization and exacerbates allergic inflammation in a mouse model of asthma. J Immunol 2019; 202(1): 119-3., 8Queener A, Jeong BM, Coden M, et al. Hyperglycemia impairs tolerance and promotes allergic inflammation in mouse models of asthma. J Immunol 2020; 204(1): 147-23.]. Hyperglycemia is assumed to be a contributor to the increased prevalence of skin sensitization, however, the clear mechanism of this phenomenon is unknown.
Skin sensitizations are growing among the general population as a result of increased exposure to environmental and industrial compounds present in toiletry and household products; the key biological events underlying skin sensitization are well-known [9Schultz TW, Dimitrova G, Dimitrov S, Mekenyan OG. The adverse outcome pathway for skin sensitisation: Moving closer to replacing animal testing. Altern Lab Anim 2016; 44(5): 453-60.
[http://dx.doi.org/10.1177/026119291604400515] [PMID: 27805828] -11The adverse outcome pathway for skin sensitisation initiated by covalent binding to proteins, OECD series on testing and assessment, No 168 2014.]. Skin sensitizer means a chemical that leads to an allergic response following skin contact. Nuclear Factor Erythroid 2-related Factor 2 (NRF2) activation is a key event triggered by skin sensitizers [12Natsch A. The Nrf2-Keap1-ARE toxicity pathway as a cellular sensor for skin sensitizers--functional relevance and a hypothesis on innate reactions to skin sensitizers. Toxicol Sci 2010; 113(2): 284-92.
[http://dx.doi.org/10.1093/toxsci/kfp228] [PMID: 19767620] , 13Helou DG, Martin SF, Pallardy M, Chollet-Martin S, Kerdine-Römer S. Nrf2 involvement in chemical-induced skin innate immunity. Front Immunol 2019; 10: 1004.
[http://dx.doi.org/10.3389/fimmu.2019.01004] [PMID: 31134077] ]. The Kelch-Like ECH-Associated Protein 1 (KEAP1)-NRF2 pathway is one of the most important cell defense systems and survival pathways against oxidative stress [14Kensler TW, Wakabayashi N, Biswal S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol 2007; 47: 89-116.
[http://dx.doi.org/10.1146/annurev.pharmtox.46.120604.141046] [PMID: 16968214] -16van der Veen JW, Gremmer ER, Vermeulen JP, van Loveren H, Ezendam J. Induction of skin sensitization is augmented in Nrf2-deficient mice. Arch Toxicol 2013; 87(4): 763-6.
[http://dx.doi.org/10.1007/s00204-012-0976-2] [PMID: 23143620] ] and is involved in dampening the induction of skin sensitization [15Limón-Pacheco J, Gonsebatt ME. The role of antioxidants and antioxidant-related enzymes in protective responses to environmentally induced oxidative stress. Mutat Res 2009; 674(1-2): 137-47.
[http://dx.doi.org/10.1016/j.mrgentox.2008.09.015] [PMID: 18955158] ]. NRF2 is anchored in the cytoplasm by KEAP1 under normal conditions. Oxidative stress induces the translocation of NRF2 into the nucleus, and then NRF2 induces gene expression via the binding to Antioxidant Response Elements (AREs) in the regulatory region of target genes, including Heme Oxygenase 1 (HMOX1) and Superoxide Dismutase (SOD) [14Kensler TW, Wakabayashi N, Biswal S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol 2007; 47: 89-116.
[http://dx.doi.org/10.1146/annurev.pharmtox.46.120604.141046] [PMID: 16968214] ]. These enzymes are a group of enzymes that catalyze the dismutation of ROS. Many reporter gene assays using a human breast cancer cell line (MCF-7 cells), a human keratinocyte cell line (HaCaT cells), and fibroblasts were developed for the evaluation of NRF2 activation [17Wang XJ, Hayes JD, Wolf CR. Generation of a stable antioxidant response element-driven reporter gene cell line and its use to show redox-dependent activation of nrf2 by cancer chemotherapeutic agents. Cancer Res 2006; 66(22): 10983-94.
[http://dx.doi.org/10.1158/0008-5472.CAN-06-2298] [PMID: 17108137] -24Jaworska J, Harol A, Kern PS, Gerberick GF. Integrating non-animal test information into an adaptive testing strategy - skin sensitization proof of concept case. ALTEX 2011; 28(3): 211-25.
[http://dx.doi.org/10.14573/altex.2011.3.211] [PMID: 21993957] ].
In this study, we investigated the involvement of glucose levels in the activation of KEAP1-NRF2 signaling by the skin sensitizers, such as eugenol, Cinnamaldehyde (CA), and 2,4-Dinitrochlorobenzene (DNCB) using MCF-7 cells and mouse BALB/3T3 fibroblasts transfected with an ARE-driven reporter plasmid. We also determined the effects of D-glucose concentration in the medium on NRF2 signaling in MCF-7 cells.
2. MATERIALS AND METHODS
2.1. Reagents
Eugenol and isopropanol were obtained from FUJIFILM Wako Pure Chemical Corporation (Osaka, Japan). CA and DNCB were obtained from Nacalai Tesque Inc. (Kyoto, Japan) and were cultured in Dulbecco’s modified Eagle’s medium-high glucose (4.5 g/L glucose) (DMEM-HG) supplemented with 10% FBS and antibiotics (100 U/mL penicillin and 100 lg/mL streptomycin).
2.2. Cell Culture
MCF-7 cells and BALB/3T3 clone A31 cells (RIKEN Cell Bank, Ibaraki, Japan) and were maintained in Dulbecco’s Modified Eagle Medium (DMEM; Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 4.5 g/L D-glucose, 10% fetal bovine serum (FBS), 1.5 g/L NaHCO3, 100 units/mL penicillin, and 100 μg/mL streptomycin (DMEM HG) at 37°C in 5% CO2 and 95% air atmosphere at 100% humidity.
2.3. Cytotoxicity Test
The cytotoxic effects of skin sensitizers on MCF-7 cells and BALB/3T3 clone A31 cells were investigated by measuring the activity of Lactate Dehydrogenase (LDH) in the culture supernatant. In 96-well plates, MCF-7 cells were cultured in phenol red-free DMEM supplemented with 1.0 g/L D-glucose and 10% FBS (DMEM NG) with the various concentrations of skin sensitizers for 24 hours, and four replicate wells were prepared for each treatment. LDH activity assay was measured using LDH Cytotoxicity Detection Kit (Dojindo, Kumamoto, Japan) as described previously [25Ogawa M, Kyoya T, Kimura T, Terada M. Effects of estrogen on fatty-acid-induced cytotoxicity in mouse Neuro-2a neural cells. Fundam Toxicol Sci 2020; 7(2): 115-21.
[http://dx.doi.org/10.2131/fts.7.115] , 26Ogawa M, Kimura T, Kitamoto J, et al. A screening system for detection of neurotoxic potency of chemicals using in vitro model. Toxicol Int in press].
2.4. Luciferase Assay
Luciferase reporter plasmid carrying ARE was constructed according to the method of Wang et al. [17Wang XJ, Hayes JD, Wolf CR. Generation of a stable antioxidant response element-driven reporter gene cell line and its use to show redox-dependent activation of nrf2 by cancer chemotherapeutic agents. Cancer Res 2006; 66(22): 10983-94.
[http://dx.doi.org/10.1158/0008-5472.CAN-06-2298] [PMID: 17108137] ]. In brief, the eight copies of the ARE (5′-GTGACAAAGCA-3′) were inserted into pGL 4.20 plasmid (Promega, Madison, WI, USA) together with an adenovirus E1b TATA sequence or simian virus 40 enhancer and early promoter, termed pAREx8-TATA and pAREx8-SV40, respectively. MCF-7 cells and BALB/3T3 clone A31 cells were cultured in Opti-MEM (Thermo Fisher Scientific) on 48-well plates, and transiently transfected with pAREx8-TATA or pAREx8-SV40 using HilyMax (Dojindo) for 24 hours, and incubated in fresh DMEM NG or DMEM HG medium, containing a test substance for an additional 24 hours. Luciferase activity was determined as described previously [27Higashimura Y, Nakajima Y, Yamaji R, et al. Up-regulation of glyceraldehyde-3-phosphate dehydrogenase gene expression by HIF-1 activity depending on Sp1 in hypoxic breast cancer cells. Arch Biochem Biophys 2011; 509(1): 1-8.
[http://dx.doi.org/10.1016/j.abb.2011.02.011] [PMID: 21338575] , 28Ogawa M, Kitano T, Kawata N, et al. Daidzein down-regulates ubiquitin-specific protease 19 expression through estrogen receptor β and increases skeletal muscle mass in young female mice. J Nutr Biochem 2017; 49: 63-70.
[http://dx.doi.org/10.1016/j.jnutbio.2017.07.017] [PMID: 28886438] ]. Transfection efficiency was normalized with Renilla luciferase expression vector (pGL 4.73 [hRluc/SV40], Promega), and data were expressed as Relative Light Units (RLU, firefly luciferase activity divided by Renilla luciferase activity).
2.5. Immunofluorescence Assay
MCF-7 cells were cultured in DMEM NG or DMEM HG medium for 24 hours, and fixed in 4% paraformaldehyde in PBS for 10 minutes, and permeabilized with 0.2% Triton X-100 in PBS for 5 minutes as described previously [26Ogawa M, Kimura T, Kitamoto J, et al. A screening system for detection of neurotoxic potency of chemicals using in vitro model. Toxicol Int in press, 29Ogawa M, Yamaji R, Higashimura Y, et al. 17β-estradiol represses myogenic differentiation by increasing ubiquitin-specific peptidase 19 through estrogen receptor α. J Biol Chem 2011; 286(48): 41455-65.
[http://dx.doi.org/10.1074/jbc.M111.276824] [PMID: 21971047] ]. Fixed cells were blocked and incubated with rabbit polyclonal NRF2 antibody (Proteintech Group Inc. Rosemont, IL, USA) at room temperature for 1 hour, followed by Alexa Fluor 594-conjugated anti-rabbit IgG (Thermo Fisher Scientific). After 1 hour, cells were counterstained with 4',6-diamidino-2-phenylindole (DAPI, Dojindo). Fluorescent images were captured using a fluorescence microscope (BX53, Olympus, Tokyo, Japan). The ratio of nuclear to cytoplasmic NRF2 fluorescence was measured.
2.6. Quantitative Real-time PCR
MCF-7 cells were cultured in DMEM NG or DMEM HG medium containing a test substance for 24 hours. Total RNA was extracted from MCF-7 cells and reverse transcribed with PrimeScript reverse transcription Reagent Kit (Takara Bio Inc., Shiga, Japan). The resultant cDNA was subjected to quantitative real-time PCR using the specific primers (Table 1). The PCR profiles consisted of denaturation at 95°C for 30 seconds, primer-annealing at 55°C for 20 seconds, and primer extension at 72°C for 20 seconds. The final primer extension was performed at 72°C for 10 minutes. The PCR was performed with SYBR Premix Ex Taq II (Takara Bio Inc.) on Thermal Cycler Dice, TP-900 (Takara Bio Inc.) in triplicate. Ct values were transformed into relative quantification data by 2-ΔΔCt method. Data were normalized to the mRNA levels of GAPDH, which encodes glyceraldehyde-3-phosphate dehydrogenase.
2.7. Superoxide Dismutase (SOD) Assay
MCF-7 cells were cultured in DMEM NG or DMEM HG medium containing a test substance for 24 hours. Cells were suspended in phosphate-buffered saline (4.3 mM Na2HPO4, 1.4 mM KH2HPO4, 137 mM NaCl, 2.7 mM KCl, pH 7.4), and sonicated for 3 minutes. Sonicated samples were then centrifugated at 10,000 x g, at 4°C, for 15 minutes. SOD activity assay was measured using SOD Assay Kit-WST (Dojindo).
2.8. Statistical Analyses
The half-maximal cytotoxic concentration (CC50) values were calculated by fitting to a standard 4-parameter logistic curve using the R (version 3.6.1). Data were statistically compared by Dunnett’s test or Student t-test using the R software (version 3.6.1). Data were expressed as mean ± S.D., and the differences were considered statistically significant at a p-value of < 0.05 (n = 3 or n = 6).
3. RESULTS
3.1. Cytotoxicity Test of Skin Sensitizers in Normal or High Glucose Conditions
Cell viability was analyzed after 24 hours of exposure to different concentrations of three skin sensitizers, eugenol, CA, and DNCB to determine which concentrations of skin sensitizers have minimal toxicity against MCF-7 cells and BALB/3T3 clone A31 cells, isopropanol was used as a negative control. In both normal and high glucose conditions, cell viabilities were decreased in a dose-dependent manner by exposing eugenol, CA, and DNCB in MCF-7 cells (Fig. 1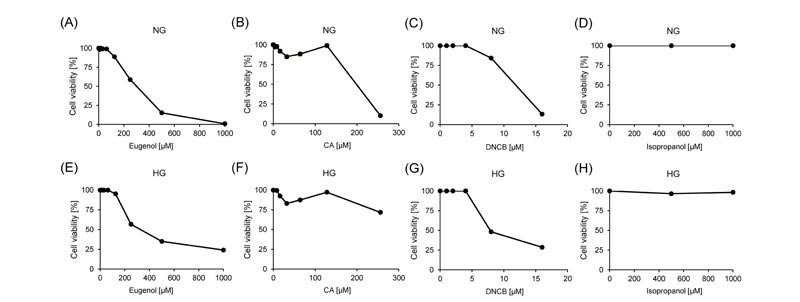 ) and BALB/3T3 clone A31 cells (Fig. 2
) and BALB/3T3 clone A31 cells (Fig. 2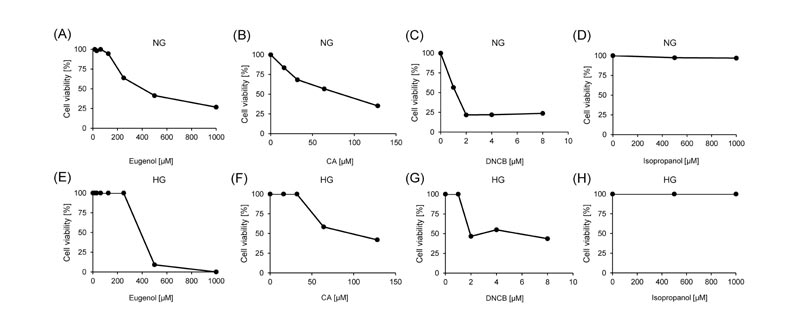 ). Isopropanol did not affect the cell viabilities of both cell lines under all conditions (Figs. 1
). Isopropanol did not affect the cell viabilities of both cell lines under all conditions (Figs. 1 and 2
and 2 ). In normal glucose conditions, the CC50 values of eugenol, CA, and DNCB were 287.8 μM, 178.4 nM, and 10.5 μM in MCF-7 cells, and 453.1 μM, 90.1 nM, and 0.9 μM in BALB/3T3 clone A31 cells, respectively (Table 2). In high glucose conditions, the CC50 values of eugenol, CA, and DNCB were 349.1 μM, 281.7 nM, and 7.3 μM in MCF-7 cells, and were 422.0 μM, 92.1 nM, and 4.6 μM in BALB/3T3 clone A31 cells, respectively (Table 2).
). In normal glucose conditions, the CC50 values of eugenol, CA, and DNCB were 287.8 μM, 178.4 nM, and 10.5 μM in MCF-7 cells, and 453.1 μM, 90.1 nM, and 0.9 μM in BALB/3T3 clone A31 cells, respectively (Table 2). In high glucose conditions, the CC50 values of eugenol, CA, and DNCB were 349.1 μM, 281.7 nM, and 7.3 μM in MCF-7 cells, and were 422.0 μM, 92.1 nM, and 4.6 μM in BALB/3T3 clone A31 cells, respectively (Table 2).
3.2. Effects of Skin Sensitizers on NRF2 Transcriptional Activity in Normal or High Glucose Conditions
MCF-7 cells were transfected with pAREx8-TATA or pAREx8-SV40 to determine the optimal reporter plasmid for evaluating NRF2 transactivation. The induction of luciferase activity by CA was 21.2-fold in pAREx8-TATA and 2.67-fold in pAREx8-SV40 (Supplementary Fig. S1 ), suggesting that the response sensitivity of pAREx8-TATA was higher than that of pAREx8-SV40. We investigated whether skin sensitizers activated the NRF2 signaling using MCF-7 cells or BALB/3T3 clone A31 cells transfected with pAREx8-TATA. Three skin sensitizers increased the luciferase activity dose-dependently in both cells under normal-glucose conditions (Figs. 3 and 4). On the other hand, the luciferase activity was increased by CA, but not eugenol and DNCB, in both cell lines under high-glucose conditions (Figs. 3
), suggesting that the response sensitivity of pAREx8-TATA was higher than that of pAREx8-SV40. We investigated whether skin sensitizers activated the NRF2 signaling using MCF-7 cells or BALB/3T3 clone A31 cells transfected with pAREx8-TATA. Three skin sensitizers increased the luciferase activity dose-dependently in both cells under normal-glucose conditions (Figs. 3 and 4). On the other hand, the luciferase activity was increased by CA, but not eugenol and DNCB, in both cell lines under high-glucose conditions (Figs. 3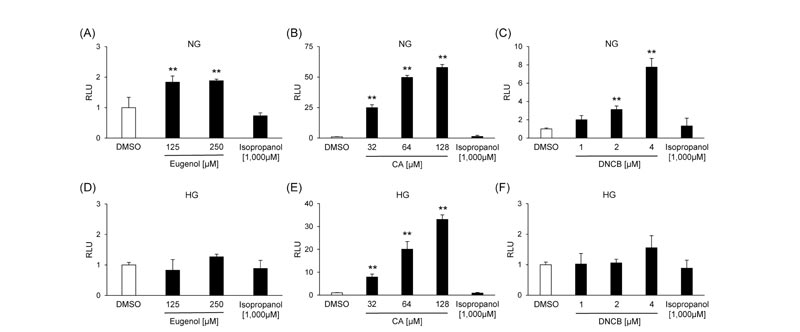 and 4
and 4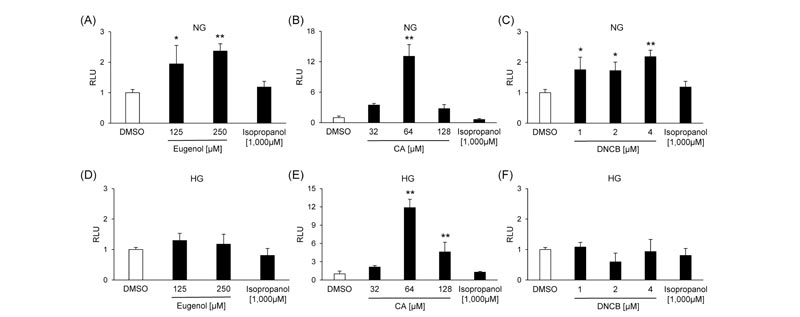 ). Isopropanol had no effects on reporter activation in both MCF-7 cells and BALB/3T3 clone A31 cells under all conditions (Figs. 3
). Isopropanol had no effects on reporter activation in both MCF-7 cells and BALB/3T3 clone A31 cells under all conditions (Figs. 3 and 4
and 4 ).
).
3.3. Effects of Glucose Concentration on the Cellular Localization of NRF2
NRF2 localization was determined by immunofluorescence assay to investigate the effects of glucose concentration on the activation of NRF2 in MCF-7 cells and BALB/3T3 clone A31 cells. Most of the NRF2 proteins were observed in the cytoplasm under both normal and high glucose conditions, but nuclear NRF2 was significantly increased under high glucose conditions compared to normal glucose conditions in MCF-7 cells (Fig. 5A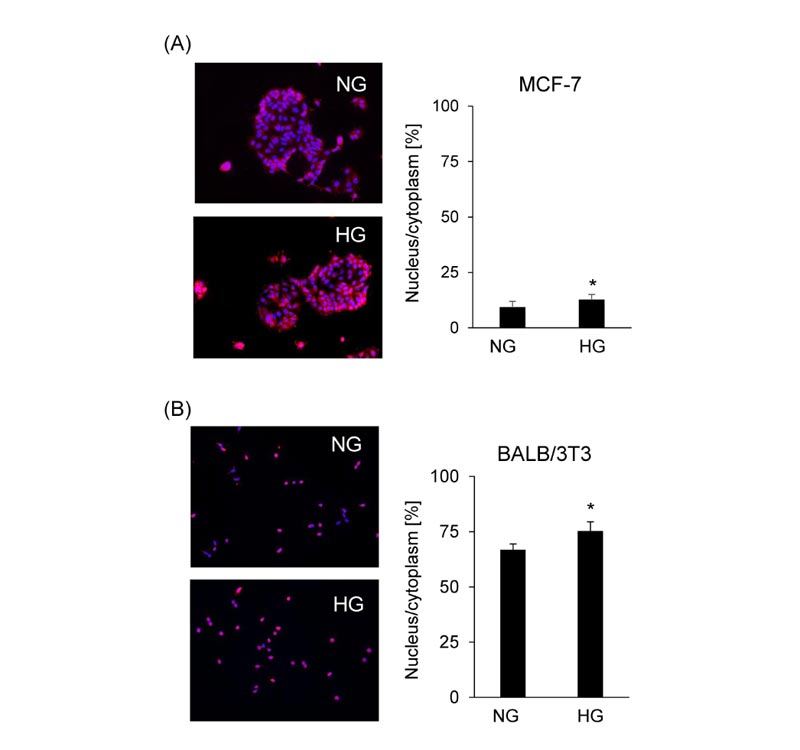 ). On the other hand, in BALB/3T3 clone A31 cells, nuclear NRF2 was also significantly increased under high glucose conditions compared to normal glucose conditions, but NRF2 proteins were almost localized at the nucleus (Fig. 5B
). On the other hand, in BALB/3T3 clone A31 cells, nuclear NRF2 was also significantly increased under high glucose conditions compared to normal glucose conditions, but NRF2 proteins were almost localized at the nucleus (Fig. 5B ), thereby, it was decided to carry out the subsequent experiments using MCF-7 cells.
), thereby, it was decided to carry out the subsequent experiments using MCF-7 cells.
3.4. Effects of Glucose Concentration on CA-Induced NRF2 Transcriptional Activity
The effect of glucose concentration on NRF2 transcriptional activity was assessed in MCF-7 cells transfected with pAREx8-TATA. High glucose conditions induced the NRF2-ARE reporter activity compared to normal glucose conditions (Fig. 6A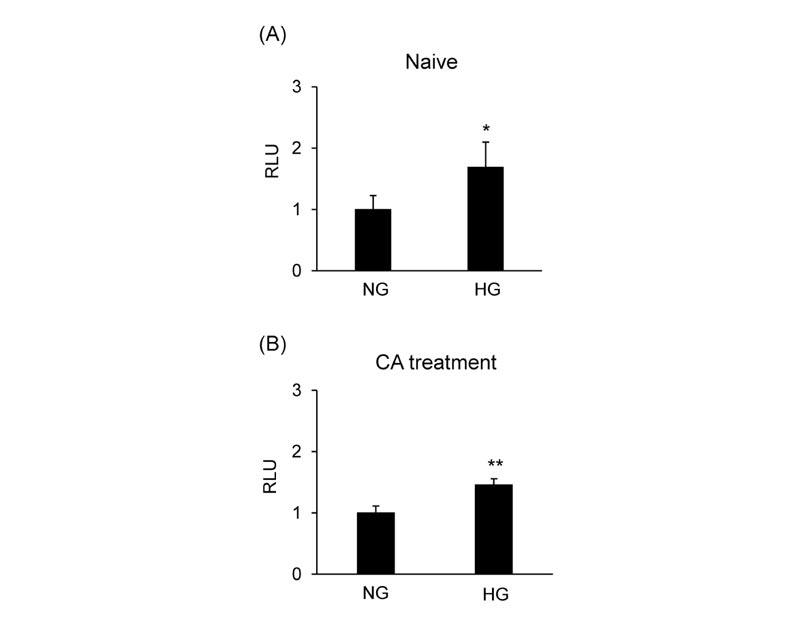 ). CA-induced NRF2 activation was significantly increased in high glucose conditions compared to normal glucose conditions (Fig. 6B
). CA-induced NRF2 activation was significantly increased in high glucose conditions compared to normal glucose conditions (Fig. 6B ).
).
3.5. Effects of Glucose Concentration on CA-Induced Anti-Oxidative Gene
The mRNA level of HMOX1 was evaluated in MCF-7 cells cultured in the medium containing various concentrations of glucose to investigate the effect of glucose concentration on the NRF2 target gene. High glucose conditions induced the level of HMOX1 mRNA compared to normal glucose conditions (Fig. 7A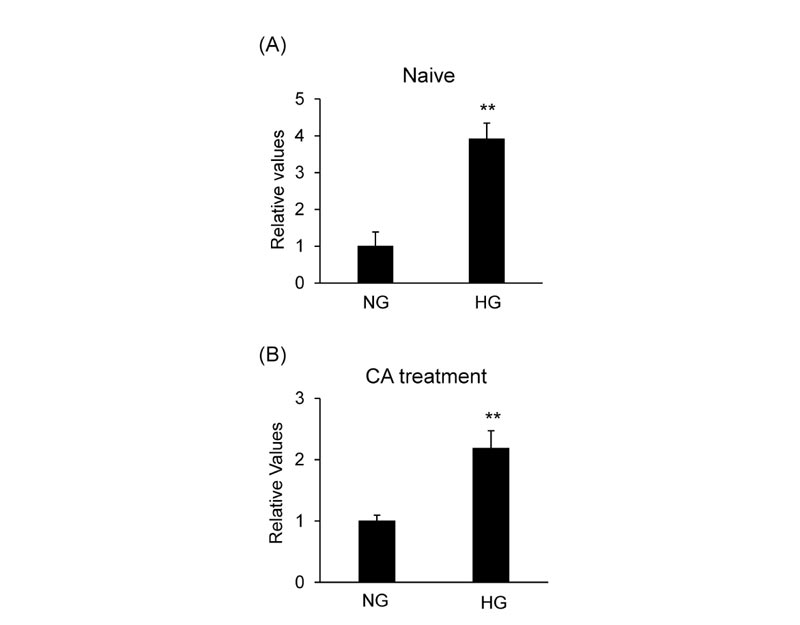 ). CA-induced HMOX1 mRNA expression was significantly increased in high glucose conditions compared to normal glucose conditions (Fig. 7B
). CA-induced HMOX1 mRNA expression was significantly increased in high glucose conditions compared to normal glucose conditions (Fig. 7B ).
).
3.6. Effects of Glucose Concentration on CA-Induced SOD-Like Activity
SOD-like activity was evaluated in MCF-7 cells cultured in the medium containing various concentrations of glucose to determine the effects of glucose on oxidative stress induced by CA. High glucose conditions induced the SOD-like activity compared to normal glucose conditions (Fig. 8A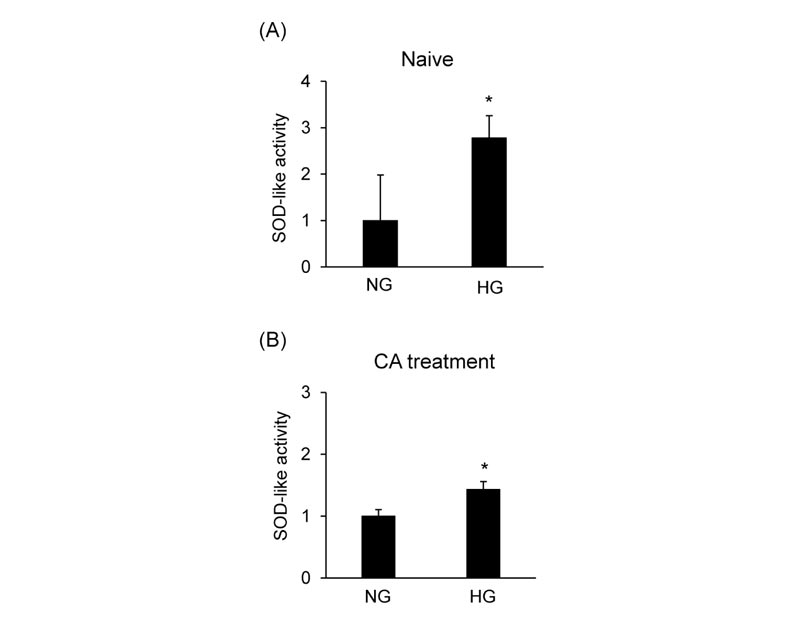 ). CA-induced SOD-like activity was significantly increased in high glucose conditions compared to normal glucose conditions (Fig. 8B
). CA-induced SOD-like activity was significantly increased in high glucose conditions compared to normal glucose conditions (Fig. 8B ).
).
4. DISCUSSION
A recent study has reported that hyperglycemia has a potentially critical role in the promotion of allergy [7Queener A, Jeong BM, Doan TC, et al. Induced hyperglycemia promotes sensitization and exacerbates allergic inflammation in a mouse model of asthma. J Immunol 2019; 202(1): 119-3., 8Queener A, Jeong BM, Coden M, et al. Hyperglycemia impairs tolerance and promotes allergic inflammation in mouse models of asthma. J Immunol 2020; 204(1): 147-23.], however, the clear mechanism of this phenomenon is unknown. KEAP1-NRF2 pathway is involved in the cellular processes in skin sensitization [12Natsch A. The Nrf2-Keap1-ARE toxicity pathway as a cellular sensor for skin sensitizers--functional relevance and a hypothesis on innate reactions to skin sensitizers. Toxicol Sci 2010; 113(2): 284-92.
[http://dx.doi.org/10.1093/toxsci/kfp228] [PMID: 19767620] ]. In this study, we demonstrated for the first time that high glucose enhanced NRF2 transcriptional activity by a skin sensitizer in vitro. This study indicates that high glucose enhanced the skin sensitizer-induced activation of NRF2. Although, the relative contribution of this mechanism to skin sensitization in vivo remains to be elucidated, diabetic patients may be more likely to develop an allergy to skin sensitizers.
DNCB, CA, and eugenol are classified as extreme, strong, and moderate sensitizers to reflect differing skin sensitization potency based on the results of local lymph node assay (LLNA) [30Test No 442A: Skin Sensitization: Local Lymph Node Assay: DA, OECD Guidelines for the Testing of Chemicals 2010.]. ARE-driven reporter assays have practical applications for detecting skin sensitizing [31Ramirez T, Mehling A, Kolle SN, et al. LuSens: A keratinocyte based ARE reporter gene assay for use in integrated testing strategies for skin sensitization hazard identification. Toxicol In Vitro 2014; 28(8): 1482-97.
[http://dx.doi.org/10.1016/j.tiv.2014.08.002] [PMID: 25172300] -35Maeda Y, Takeyoshi M, Chuma T, Iwata H. α-Sens: The improved ARE-Nrf2-based sensitization screening assay using normalized transcriptional activity. Toxicology 2020; 439152476
[http://dx.doi.org/10.1016/j.tox.2020.152476] [PMID: 32335162] ]. We demonstrated that the ranking of the activities was DNCB > CA > eugenol in MCF-7 cells or BALB/3T3 clone A31 cells transfected with an ARE reporter plasmid under normal glucose conditions. Therefore, these indicate that our two assays can estimate the potency class (weak, moderate, strong, or extreme) of skin sensitizers similar to previous ARE-driven reporter assays.
HMOX1 mRNA and SOD-like activity in high glucose conditions were higher than in normal glucose conditions in both the absence and presence of CA. Furthermore, glucose induced the nuclear translocation and transactivation of NRF2 in MCF-7 cells 24 hours after the treatment. The production of H2O2 is induced by high glucose concentration within 24 hours in vitro [36Ouedraogo R, Wu X, Xu SQ, et al. Adiponectin suppression of high-glucose-induced reactive oxygen species in vascular endothelial cells: Evidence for involvement of a cAMP signaling pathway. Diabetes 2006; 55(6): 1840-6.
[http://dx.doi.org/10.2337/db05-1174] [PMID: 16731851] ]. High glucose increases the percentage of 8-hydroxy-2'-deoxyguanosine-positive cells compared to the control at 8 hours after the treatment [37Kim JW, Park SY, You YH, et al. Suppression of ROS production by exendin-4 in PSC attenuates the high glucose-induced islet fibrosis. PLoS One 2016; 11(12)e0163187
[http://dx.doi.org/10.1371/journal.pone.0163187] [PMID: 27977690] ]. Under oxidative stress, the conformation of KEAP1 is modified, leading to NRF2 release, which translocates to the nucleus [38Hsieh CY, Hsiao HY, Wu WY, et al. Regulation of shear-induced nuclear translocation of the Nrf2 transcription factor in endothelial cells. J Biomed Sci 2009; 16(1): 12.
[http://dx.doi.org/10.1186/1423-0127-16-12] [PMID: 19272177] ]. Hyperglycemia induces NRF2 activation indirectly via ROS production [39Li H, Yao W, Irwin MG, et al. Adiponectin ameliorates hyperglycemia-induced cardiac hypertrophy and dysfunction by concomitantly activating Nrf2 and Brg1. Free Radic Biol Med 2015; 84: 311-21.
[http://dx.doi.org/10.1016/j.freeradbiomed.2015.03.007] [PMID: 25795513] , 40Sharma A, Rizky L, Stefanovic N, et al. The nuclear factor (erythroid-derived 2)-like 2 (Nrf2) activator dh404 protects against diabetes-induced endothelial dysfunction. Cardiovasc Diabetol 2017; 16(1): 33.
[http://dx.doi.org/10.1186/s12933-017-0513-y] [PMID: 28253885] ]. Therefore, this indicates that glucose causes oxidative stress and assists the CA-induced NRF2 activation in vitro.
NRF2 facilitates the repair of radiation-induced DNA damage in a ROS- independent manner [41Jayakumar S, Pal D, Sandur SK. Nrf2 facilitates repair of radiation induced DNA damage through homologous recombination repair pathway in a ROS independent manner in cancer cells. Mutat Res 2015; 779: 33-45.
[http://dx.doi.org/10.1016/j.mrfmmm.2015.06.007] [PMID: 26133502] ]. Extreme high glucose (concentrations above 10 g/L) induces cytotoxic, genotoxic, and apoptotic effects on MCF-7 cells [42Tchounwou CK, Yedjou CG, Farah I, Tchounwou PB. D-Glucose-induced cytotoxic, genotoxic, and apoptotic effects on human breast adenocarcinoma (MCF-7) cells. J Cancer Sci Ther 2014; 6: 156-60.
[http://dx.doi.org/10.4172/1948-5956.1000265] [PMID: 25506409] ]. It was hypothesized that NRF2 was activated in response to cell damage by glucose, however, the cytotoxicity of skin sensitizers in MCF-7 cells and BALB/3T3 clone A31 cells was not dependent on the glucose concentration in the medium in this study. Blood glucose concentrations are usually in the range between 2.0 and 4.5 g/L in diabetic patients [43Fitrullah , Rousdy A. Fitrullah; Rousdy, A. Effectiveness of acupressure at the zusanli (ST-36) acupoint as a comfortable treatment for diabetes mellitus: A pilot study in Indonesia. J Acupunct Meridian Stud 2017; 10(2): 96-103.
[http://dx.doi.org/10.1016/j.jams.2016.12.003] [PMID: 28483191] , 44Patel BJ, Dave B, Dave D, Karmakar P, Shah M, Sarvaiya B. Comparison and correlation of glucose levels in serum and saliva of noth diabetic and non-diabetic patients. J Int Oral Health 2015; 7(8): 70-6.
[PMID: 26464543] ]. Therefore, these findings suggest that hyperglycemia-promoted oxidative stress probably leads to the activation of NRF2 without cytotoxicity in diabetes.
This study showed that the response sensitivity of MCF-7 cells was higher than that of BALB/3T3 clone A31 cells. Several skin sensitizers were not able to be evaluated in BALB/3T3 clone A31 cells. NRF2 is basally activated in human and mouse fibroblasts [45Paupe V, Dassa EP, Goncalves S, et al. Impaired nuclear Nrf2 translocation undermines the oxidative stress response in Friedreich ataxia. PLoS One 2009; 4(1)e4253
[http://dx.doi.org/10.1371/journal.pone.0004253] [PMID: 19158945] , 46Daiana S, Ana R, Victoria M, Julian N, Enrique M, Omar C. A major role for Nrf2 transcription factors in cell transformation by KSHV encoded oncogenes. bioRxiv 2019.678342]; by contrast, the basal activation of NRF2 is not observed in MCF-7 cells [47Probst BL, McCauley L, Trevino I, Wigley WC, Ferguson DA. Cancer cell Growth is differentially affected by constitutive activation of NRF2 by KEAP1 deletion and pharmacological activation of NRF2 by the synthetic triterpenoid, RTA 405. PLoS One 2015; 10(8)e0135257
[http://dx.doi.org/10.1371/journal.pone.0135257] [PMID: 26301506] ]. We also demonstrated that the nuclear ratio of NRF2 in BALB/3T3 clone A31 was higher than in MCF-7 cells. Thus, this indicates that the response to stimulation by skin sensitizers in MCF-7 cells is higher than in BALB/3T3 clone A31 cells.
Our reporter assays showed that eugenol and DNCB induced the reporter activity in both MCF-7 cells and BALB/3T3 clone A31 cells under normal glucose conditions, but not high glucose conditions. Furthermore, the luciferase activity induced by CA showed higher fold changes compared to vehicle control under normal glucose conditions than high glucose conditions. We also demonstrated that nuclear NRF2 levels in normal glucose conditions were lower than in high glucose conditions. In short, NRF2 activity under basal conditions was maintained at low levels under normal conditions. For ARE-driven reporter gene assays, our results propose that the lower concentrations of glucose in the medium lead to enhanced detection sensitivity for skin sensitizers.
CONCLUSION
We demonstrated for the first time that glucose enhanced skin sensitizers-induced NRF2 transcriptional activity and SOD-like activity in vitro. This indicate that oxidative stress caused by hyperglycemia additionally induced the activation of NRF2 signaling by skin sensitization. Further studies are needed to investigate the effects of blood glucose levels on skin sensitization in vivo.
LIST OF ABBREVIATIONS
| ARE | = Antioxidant Response Element |
| CC50 | = Half-Maximal Cytotoxic Concentration |
| CA | = Cinnamaldehyde |
| CS | = Dextran-Coated Charcoal-Stripped |
| DMEM | = Dulbecco’s Modified Eagle Medium |
| DNCB | = 2,4-Dinitrochlorobenzene |
| GAPDH | = Glyceraldehyde-3-Phosphate Dehydrogenase |
| HG | = High Glucose |
| KEAP1 | = Kelch-like ECH-associated Protein 1 |
| LDH | = Lactate Dehydrogenase |
| LLNA | = Local Lymph Node Assay |
| HMOX1 | = Heme Oxygenase 1 |
| NG | = Normal Glucose |
| NRF2 | = Nuclear Factor Erythroid 2-Related Factor 2 |
| ROS | = Reactive Oxygen Species |
| SOD | = Superoxide Dismutase |
AUTHOR’S CONTRIBUTION
The article has been written by Takeo Takeda and Masahiro Ogawa. The data has been analyzed by Takeo Takeda, Junya Kitamoto, Takahiro Kyoya, and Megumi Terada. The study has been designed by Takeda Takeo and Masahiro Ogawa. The data has been collected by Takeda Takeo.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
HUMAN AND ANIMAL RIGHTS
No animals/humans were used for studies that are basis of this research.
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIAL
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
We are deeply grateful to Dr. Tomoya Kitakaze in Kobe University for the valuable advice. We thank Ms. Masami Hori and Ms. Hiroe Muramatsu in Kumiai Chemical Industry Co. Ltd for technical assistance. We also thank Mr. Ryota Kikuchi, a software developer, for the establishment of a statistical analysis system using R software.
SUPPLEMENTARY MATERIAL
Supplementary material is available on the publishers web site along with the published article.
REFERENCES
| [1] | Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res 2010; 107(9): 1058-70. [http://dx.doi.org/10.1161/CIRCRESAHA.110.223545] [PMID: 21030723] |
| [2] | Oguntibeju OO. Type 2 diabetes mellitus, oxidative stress and inflammation: Examining the links. Int J Physiol Pathophysiol Pharmacol 2019; 11(3): 45-63. [PMID: 31333808] |
| [3] | King GL, Loeken MR. Hyperglycemia-induced oxidative stress in diabetic complications. Histochem Cell Biol 2004; 122(4): 333-8. [http://dx.doi.org/10.1007/s00418-004-0678-9] [PMID: 15257460] |
| [4] | Du X, Matsumura T, Edelstein D, et al. Inhibition of GAPDH activity by poly(ADP-ribose) polymerase activates three major pathways of hyperglycemic damage in endothelial cells. J Clin Invest 2003; 112(7): 1049-57. [http://dx.doi.org/10.1172/JCI18127] [PMID: 14523042] |
| [5] | Bach JF. The effect of infections on susceptibility to autoimmune and allergic diseases. N Engl J Med 2002; 347(12): 911-20. [http://dx.doi.org/10.1056/NEJMra020100] [PMID: 12239261] |
| [6] | Tosca MA, Silvestri M, Olcese R, et al. Allergic sensitization and symptoms, body mass index, and respiratory function in children with type 1 diabetes mellitus. Ann Allergy Asthma Immunol 2012; 108(2): 128-9. [http://dx.doi.org/10.1016/j.anai.2011.12.002] [PMID: 22289736] |
| [7] | Queener A, Jeong BM, Doan TC, et al. Induced hyperglycemia promotes sensitization and exacerbates allergic inflammation in a mouse model of asthma. J Immunol 2019; 202(1): 119-3. |
| [8] | Queener A, Jeong BM, Coden M, et al. Hyperglycemia impairs tolerance and promotes allergic inflammation in mouse models of asthma. J Immunol 2020; 204(1): 147-23. |
| [9] | Schultz TW, Dimitrova G, Dimitrov S, Mekenyan OG. The adverse outcome pathway for skin sensitisation: Moving closer to replacing animal testing. Altern Lab Anim 2016; 44(5): 453-60. [http://dx.doi.org/10.1177/026119291604400515] [PMID: 27805828] |
| [10] | Rossi LH, Ezendam J. Predicting chemically induced skin sensitization by using in chemico / in vitro methods. Methods Mol Biol 2018; 1800: 485-504. [http://dx.doi.org/10.1007/978-1-4939-7899-1_22] [PMID: 29934907] |
| [11] | The adverse outcome pathway for skin sensitisation initiated by covalent binding to proteins, OECD series on testing and assessment, No 168 2014. |
| [12] | Natsch A. The Nrf2-Keap1-ARE toxicity pathway as a cellular sensor for skin sensitizers--functional relevance and a hypothesis on innate reactions to skin sensitizers. Toxicol Sci 2010; 113(2): 284-92. [http://dx.doi.org/10.1093/toxsci/kfp228] [PMID: 19767620] |
| [13] | Helou DG, Martin SF, Pallardy M, Chollet-Martin S, Kerdine-Römer S. Nrf2 involvement in chemical-induced skin innate immunity. Front Immunol 2019; 10: 1004. [http://dx.doi.org/10.3389/fimmu.2019.01004] [PMID: 31134077] |
| [14] | Kensler TW, Wakabayashi N, Biswal S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol 2007; 47: 89-116. [http://dx.doi.org/10.1146/annurev.pharmtox.46.120604.141046] [PMID: 16968214] |
| [15] | Limón-Pacheco J, Gonsebatt ME. The role of antioxidants and antioxidant-related enzymes in protective responses to environmentally induced oxidative stress. Mutat Res 2009; 674(1-2): 137-47. [http://dx.doi.org/10.1016/j.mrgentox.2008.09.015] [PMID: 18955158] |
| [16] | van der Veen JW, Gremmer ER, Vermeulen JP, van Loveren H, Ezendam J. Induction of skin sensitization is augmented in Nrf2-deficient mice. Arch Toxicol 2013; 87(4): 763-6. [http://dx.doi.org/10.1007/s00204-012-0976-2] [PMID: 23143620] |
| [17] | Wang XJ, Hayes JD, Wolf CR. Generation of a stable antioxidant response element-driven reporter gene cell line and its use to show redox-dependent activation of nrf2 by cancer chemotherapeutic agents. Cancer Res 2006; 66(22): 10983-94. [http://dx.doi.org/10.1158/0008-5472.CAN-06-2298] [PMID: 17108137] |
| [18] | Emter R, Ellis G, Natsch A. Performance of a novel keratinocyte-based reporter cell line to screen skin sensitizers in vitro. Toxicol Appl Pharmacol 2010; 245(3): 281-90. [http://dx.doi.org/10.1016/j.taap.2010.03.009] [PMID: 20307559] |
| [19] | Chen YT, Shi D, Yang D, Yan B. Antioxidant sulforaphane and sensitizer trinitrobenzene sulfonate induce carboxylesterase-1 through a novel element transactivated by nuclear factor-E2 related factor-2. Biochem Pharmacol 2012; 84(6): 864-71. [http://dx.doi.org/10.1016/j.bcp.2012.06.025] [PMID: 22776248] |
| [20] | Natsch A, Emter R. Skin sensitizers induce antioxidant response element dependent genes: application to the in vitro testing of the sensitization potential of chemicals. Toxicol Sci 2008; 102(1): 110-9. [http://dx.doi.org/10.1093/toxsci/kfm259] [PMID: 17932397] |
| [21] | Han EH, Hwang YP, Jeong TC, Lee SS, Shin JG, Jeong HG. Eugenol inhibit 7,12-dimethylbenz[a]anthracene-induced genotoxicity in MCF-7 cells: Bifunctional effects on CYP1 and NAD(P)H:quinone oxidoreductase. FEBS Lett 2007; 581(4): 749-56. [http://dx.doi.org/10.1016/j.febslet.2007.01.044] [PMID: 17275817] |
| [22] | Al Wafai R, El-Rabih W, Katerji M, et al. Chemosensitivity of MCF-7 cells to eugenol: release of cytochrome-c and lactate dehydrogenase. Sci Rep 2017; 7: 43730. [http://dx.doi.org/10.1038/srep43730] [PMID: 28272477] |
| [23] | Reisinger K, Hoffmann S, Alépée N, et al. Systematic evaluation of non-animal test methods for skin sensitisation safety assessment. Toxicol In Vitro 2015; 29(1): 259-70. [http://dx.doi.org/10.1016/j.tiv.2014.10.018] [PMID: 25448812] |
| [24] | Jaworska J, Harol A, Kern PS, Gerberick GF. Integrating non-animal test information into an adaptive testing strategy - skin sensitization proof of concept case. ALTEX 2011; 28(3): 211-25. [http://dx.doi.org/10.14573/altex.2011.3.211] [PMID: 21993957] |
| [25] | Ogawa M, Kyoya T, Kimura T, Terada M. Effects of estrogen on fatty-acid-induced cytotoxicity in mouse Neuro-2a neural cells. Fundam Toxicol Sci 2020; 7(2): 115-21. [http://dx.doi.org/10.2131/fts.7.115] |
| [26] | Ogawa M, Kimura T, Kitamoto J, et al. A screening system for detection of neurotoxic potency of chemicals using in vitro model. Toxicol Int in press |
| [27] | Higashimura Y, Nakajima Y, Yamaji R, et al. Up-regulation of glyceraldehyde-3-phosphate dehydrogenase gene expression by HIF-1 activity depending on Sp1 in hypoxic breast cancer cells. Arch Biochem Biophys 2011; 509(1): 1-8. [http://dx.doi.org/10.1016/j.abb.2011.02.011] [PMID: 21338575] |
| [28] | Ogawa M, Kitano T, Kawata N, et al. Daidzein down-regulates ubiquitin-specific protease 19 expression through estrogen receptor β and increases skeletal muscle mass in young female mice. J Nutr Biochem 2017; 49: 63-70. [http://dx.doi.org/10.1016/j.jnutbio.2017.07.017] [PMID: 28886438] |
| [29] | Ogawa M, Yamaji R, Higashimura Y, et al. 17β-estradiol represses myogenic differentiation by increasing ubiquitin-specific peptidase 19 through estrogen receptor α. J Biol Chem 2011; 286(48): 41455-65. [http://dx.doi.org/10.1074/jbc.M111.276824] [PMID: 21971047] |
| [30] | Test No 442A: Skin Sensitization: Local Lymph Node Assay: DA, OECD Guidelines for the Testing of Chemicals 2010. |
| [31] | Ramirez T, Mehling A, Kolle SN, et al. LuSens: A keratinocyte based ARE reporter gene assay for use in integrated testing strategies for skin sensitization hazard identification. Toxicol In Vitro 2014; 28(8): 1482-97. [http://dx.doi.org/10.1016/j.tiv.2014.08.002] [PMID: 25172300] |
| [32] | Ramirez T, Stein N, Aumann A, et al. Intra- and inter-laboratory reproducibility and accuracy of the LuSens assay: A reporter gene-cell line to detect keratinocyte activation by skin sensitizers. Toxicol In Vitro 2016; 32: 278-86. [http://dx.doi.org/10.1016/j.tiv.2016.01.004] [PMID: 26796489] |
| [33] | Bergal M, Puginier M, Gerbeix C, et al. In vitro testing strategy for assessing the skin sensitizing potential of “difficult to test” cosmetic ingredients. Toxicol In Vitro 2020; 65104781 [http://dx.doi.org/10.1016/j.tiv.2020.104781] [PMID: 32001296] |
| [34] | Nishijo T, Miyazawa M, Saito K, Otsubo Y, Mizumachi H, Sakaguchi H. Sensitivity of KeratinoSensTM and h-CLAT for detecting minute amounts of sensitizers to evaluate botanical extract. J Toxicol Sci 2019; 44(1): 13-21. [http://dx.doi.org/10.2131/jts.44.13] [PMID: 30626776] |
| [35] | Maeda Y, Takeyoshi M, Chuma T, Iwata H. α-Sens: The improved ARE-Nrf2-based sensitization screening assay using normalized transcriptional activity. Toxicology 2020; 439152476 [http://dx.doi.org/10.1016/j.tox.2020.152476] [PMID: 32335162] |
| [36] | Ouedraogo R, Wu X, Xu SQ, et al. Adiponectin suppression of high-glucose-induced reactive oxygen species in vascular endothelial cells: Evidence for involvement of a cAMP signaling pathway. Diabetes 2006; 55(6): 1840-6. [http://dx.doi.org/10.2337/db05-1174] [PMID: 16731851] |
| [37] | Kim JW, Park SY, You YH, et al. Suppression of ROS production by exendin-4 in PSC attenuates the high glucose-induced islet fibrosis. PLoS One 2016; 11(12)e0163187 [http://dx.doi.org/10.1371/journal.pone.0163187] [PMID: 27977690] |
| [38] | Hsieh CY, Hsiao HY, Wu WY, et al. Regulation of shear-induced nuclear translocation of the Nrf2 transcription factor in endothelial cells. J Biomed Sci 2009; 16(1): 12. [http://dx.doi.org/10.1186/1423-0127-16-12] [PMID: 19272177] |
| [39] | Li H, Yao W, Irwin MG, et al. Adiponectin ameliorates hyperglycemia-induced cardiac hypertrophy and dysfunction by concomitantly activating Nrf2 and Brg1. Free Radic Biol Med 2015; 84: 311-21. [http://dx.doi.org/10.1016/j.freeradbiomed.2015.03.007] [PMID: 25795513] |
| [40] | Sharma A, Rizky L, Stefanovic N, et al. The nuclear factor (erythroid-derived 2)-like 2 (Nrf2) activator dh404 protects against diabetes-induced endothelial dysfunction. Cardiovasc Diabetol 2017; 16(1): 33. [http://dx.doi.org/10.1186/s12933-017-0513-y] [PMID: 28253885] |
| [41] | Jayakumar S, Pal D, Sandur SK. Nrf2 facilitates repair of radiation induced DNA damage through homologous recombination repair pathway in a ROS independent manner in cancer cells. Mutat Res 2015; 779: 33-45. [http://dx.doi.org/10.1016/j.mrfmmm.2015.06.007] [PMID: 26133502] |
| [42] | Tchounwou CK, Yedjou CG, Farah I, Tchounwou PB. D-Glucose-induced cytotoxic, genotoxic, and apoptotic effects on human breast adenocarcinoma (MCF-7) cells. J Cancer Sci Ther 2014; 6: 156-60. [http://dx.doi.org/10.4172/1948-5956.1000265] [PMID: 25506409] |
| [43] | Fitrullah , Rousdy A. Fitrullah; Rousdy, A. Effectiveness of acupressure at the zusanli (ST-36) acupoint as a comfortable treatment for diabetes mellitus: A pilot study in Indonesia. J Acupunct Meridian Stud 2017; 10(2): 96-103. [http://dx.doi.org/10.1016/j.jams.2016.12.003] [PMID: 28483191] |
| [44] | Patel BJ, Dave B, Dave D, Karmakar P, Shah M, Sarvaiya B. Comparison and correlation of glucose levels in serum and saliva of noth diabetic and non-diabetic patients. J Int Oral Health 2015; 7(8): 70-6. [PMID: 26464543] |
| [45] | Paupe V, Dassa EP, Goncalves S, et al. Impaired nuclear Nrf2 translocation undermines the oxidative stress response in Friedreich ataxia. PLoS One 2009; 4(1)e4253 [http://dx.doi.org/10.1371/journal.pone.0004253] [PMID: 19158945] |
| [46] | Daiana S, Ana R, Victoria M, Julian N, Enrique M, Omar C. A major role for Nrf2 transcription factors in cell transformation by KSHV encoded oncogenes. bioRxiv 2019.678342 |
| [47] | Probst BL, McCauley L, Trevino I, Wigley WC, Ferguson DA. Cancer cell Growth is differentially affected by constitutive activation of NRF2 by KEAP1 deletion and pharmacological activation of NRF2 by the synthetic triterpenoid, RTA 405. PLoS One 2015; 10(8)e0135257 [http://dx.doi.org/10.1371/journal.pone.0135257] [PMID: 26301506] |











