- Home
- About Journals
-
Information for Authors/ReviewersEditorial Policies
Publication Fee
Publication Cycle - Process Flowchart
Online Manuscript Submission and Tracking System
Publishing Ethics and Rectitude
Authorship
Author Benefits
Reviewer Guidelines
Guest Editor Guidelines
Peer Review Workflow
Quick Track Option
Copyediting Services
Bentham Open Membership
Bentham Open Advisory Board
Archiving Policies
Fabricating and Stating False Information
Post Publication Discussions and Corrections
Editorial Management
Advertise With Us
Funding Agencies
Rate List
Kudos
General FAQs
Special Fee Waivers and Discounts
- Contact
- Help
- About Us
- Search

The Open Virology Journal
(Discontinued)
ISSN: 1874-3579 ― Volume 15, 2021
Protection Efficacy of C5A Against Vaginal and Rectal HIV Challenges in Humanized Mice
Philippe A. Gallay1, *, Udayan Chatterji1, Aaron Kirchhoff1, Angel Gandarilla1, Richard B. Pyles2, Marc M. Baum3, John A. Moss3
Abstract
Introduction:
In the absence of a vaccine, there is an urgent need for the identification of effective agents that prevent HIV transmission in uninfected individuals. Non-vaccine Biomedical Prevention (nBP) methods, such as topical or systemic pre-exposure prophylaxis (PrEP), are promising strategies to slow down the spread of AIDS.
Methods:
In this study, we investigated the microbicidal efficacy of the viral membrane-disrupting amphipathic SWLRDIWDWICEVLSDFK peptide called C5A. We chose the bone marrow/liver/thymus (BLT) humanized mouse model as vaginal and rectal HIV transmission models.
Results:
We found that the topical administration of C5A offers complete protection against vaginal and rectal HIV challenges in humanized mice. After demonstrating that C5A blocks genital HIV transmission in humanized mice, we examined the molecular requirements for its microbicidal property. We found that the removal of four amino acids on either end of C5A does not diminish its microbicidal efficacy. However, the removal of four amino acids at both the ends, abolishes its capacity to prevent vaginal or rectal HIV transmission, suggesting that the length of the peptide is a critical parameter for the microbicidal activity of C5A. Moreover, we demonstrated that the amphipathicity of the helical peptide as well as its hydrophobic surface represents key factors for the microbicidal activity of C5A in humanized mice.
Conclusion:
With its noncellular cytotoxic activity, its property of neutralizing both HSV and HIV, and its unique mechanism of action that disrupts the stability of the viral membrane, C5A represents an attractive multipurpose microbicidal candidate to be combined with other anti-HIV agents including antiretrovirals.
Article Information
Identifiers and Pagination:
Year: 2018Volume: 12
First Page: 1
Last Page: 13
Publisher Id: TOVJ-12-1
DOI: 10.2174/1874357901812010001
Article History:
Received Date: 15/08/2017Revision Received Date: 12/12/2017
Acceptance Date: 29/01/2018
Electronic publication date: 28/02/2018
Collection year: 2018
open-access license: This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International Public License (CC-BY 4.0), a copy of which is available at: https://creativecommons.org/licenses/by/4.0/legalcode. This license permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
* Address correspondence to this author at the Department of Immunology & Microbiology, The Scripps Research Institute, Philippe A. Gallay , IMM-9, 10550 N. Torrey Pines Rd, La Jolla, USA; Tel: (858) 784-8180; E-mail: gallay@scripps.edu
| Open Peer Review Details | |||
|---|---|---|---|
| Manuscript submitted on 15-08-2017 |
Original Manuscript | Protection Efficacy of C5A Against Vaginal and Rectal HIV Challenges in Humanized Mice | |
1. INTRODUCTION
Given that there is currently no vaccine, there is an immediate need for effective agents to decrease the significant number of new HIV transmissions, recently estimated to be at ~7000 new infections/day globally [12010.WHO-UNAIDS Report on the Global AIDS Epidemic http://www.unaids.org/ globalreport/global_report.htm]. Non-vaccine Biomedical Prevention (NBP) methods in the form of topical or systemic PrEP are attractive strategies to control AIDS dissemination [2Cohen MS, Gay C, Kashuba AD, Blower S, Paxton L. Narrative review: Antiretroviral therapy to prevent the sexual transmission of HIV-1. Ann Intern Med 2007; 146: 591-601.
[http://dx.doi.org/10.7326/0003-4819-146-8-200704170-00010] -15Turpin JA. Topical microbicides to prevent the transmission of HIV: Formulation gaps and challenges. Drug Deliv Transl Res 2011; 1: 194-200.
[http://dx.doi.org/10.1007/s13346-011-0034-2] ]. Several clinical trials, based on regimens of tenofovir (TFV), frequently in combination with emtricitabine (FTC), provided evidence that PrEP could significantly reduce HIV infection in individuals [16Abdool Karim Q, Abdool Karim SS, Frohlich JA, et al. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science 2010; 329: 1168-74.
[http://dx.doi.org/10.1126/science.1193748] -24McCormack S, Dunn DT, Desai M, et al. Pre-exposure prophylaxis to prevent the acquisition of HIV-1 infection (PROUD): Effectiveness results from the pilot phase of a pragmatic open-label randomised Trial. Lancet 2016; 387: 53-60.
[http://dx.doi.org/10.1016/S0140-6736(15)00056-2] ].The CAPRISA 004 trial provided the first demonstration that a topical microbicide could prevent HIV transmission in humans. Specifically, a 1% tenofovir (TVF) gel used pericoitally decreased the incidence of HIV transmission in South African women by 39% [16Abdool Karim Q, Abdool Karim SS, Frohlich JA, et al. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science 2010; 329: 1168-74.
[http://dx.doi.org/10.1126/science.1193748] ]. However, two additional trials with 1% tenofovir gel with pericoital (VOICE) [25Delany-Moretlwe S, Smith E, Myer L, et al. FACTS 001: Characteristics of participants enrolled in a phase III randomised controlled trial of tenofovir gel for prevention of HIV-1 and HSV-2. AIDS Res Hum Retroviruses 2014; 30: A281-2.
[http://dx.doi.org/10.1089/aid.2014.5637.abstract] ] and daily (FACTS) [26Marrazzo JM, Ramjee G, Richardson BA, Gomez K, Mgodi N, Nair G, et al. Tenofovir-based preexposure prophylaxis for HIV infection among african women. Obstet Gynecol Surv 2015; 70: 444-6.
[http://dx.doi.org/10.1097/01.ogx.0000466878.37011.6f] ] dosing regimens failed to provide efficacy against new sexual HIV infections. In the ASPIRE trial of a monthly intravaginal ring delivering dapivirine, incidence of HIV infection was significantly reduced for women who wore the ring consistently, but low overall efficacy (27% risk reduction) resulted from poor adherence in certain sub-groups, particularly among younger women [27Baeten JM, Palanee-Phillips T, Brown ER, Schwartz K, Soto-Torres LE, Govender V, et al. Use of a vaginal ring containing dapivirine for HIV-1 prevention in women. N Engl J Med 2016; 375: 2121-32.
[http://dx.doi.org/10.1056/NEJMoa1506110] ]. Indeed, poor adherence to the prophylaxis regimens may be a primary factor in this lack of efficacy; although additional factors cannot be ruled out to account for the disparate range
of efficacy in the results, as indicated in a randomized pharmacokinetic crossover study [28Hendrix CW. Exploring concentration response in HIV pre-exposure prophylaxis to optimize clinical care and trial design. Cell 2013; 155: 515-8.
[http://dx.doi.org/10.1016/j.cell.2013.09.030] ], where the tissue concentration advantage (>100x) seen topically as compared to oral dosing was not reflected in the seroconversion data of the CAPRISA and VOICE trials. This may indicate that factors beyond the purely antiviral effect may reduce topical PrEP efficacy. Potential factors include concentration-dependent tissue toxicity from TFV, tenofovir-diphosphate (TFV-DP) effects, or dose frequency-dependent effects from the gel itself. Issues such as these may be difficult to adequately assess, and may be outside the scope of standard safety evaluation. In any case, the conflicting trial results certainly point out the multiple factors at work in the interactions between HIV and the host at the mucosal surfaces during initiation of infection, and highlight an urgent need for suitable in vivo model systems that can illuminate the various possible contributions to the contradictory clinical results.
The use of microbicides in studies, by the fact of their tissue-specific nature, requires the use of animal models [3Derdelinckx I, Wainberg MA, Lange JM, Hill A, Halima Y, Boucher CA. Criteria for drugs used in pre-exposure prophylaxis trials against HIV infection. PLoS Med 2006; 3: e454.
[http://dx.doi.org/10.1371/journal.pmed.0030454] , 23Marcus JL, Hurley LB, Hare CB, et al. Preexposure prophylaxis for HIV prevention in a large integrated health care system: Adherence, renal safety, and discontinuation. J Acquir Immune Defic Syndr 2016; 73: 540-6.
[http://dx.doi.org/10.1097/QAI.0000000000001129] -25Delany-Moretlwe S, Smith E, Myer L, et al. FACTS 001: Characteristics of participants enrolled in a phase III randomised controlled trial of tenofovir gel for prevention of HIV-1 and HSV-2. AIDS Res Hum Retroviruses 2014; 30: A281-2.
[http://dx.doi.org/10.1089/aid.2014.5637.abstract] ]. One animal model often used in the study of vaginal HIV transmission is the Rhesus macaque; in this case, the animals are infected with either Simian Immunodeficiency Virus (SIV) or SIV/HIV (SHIV) chimeric viruses [29Veazey RS, Springer MS, Marx PA, Dufour J, Klasse PJ, Moore JP. Protection of macaques from vaginal SHIV challenge by an orally delivered CCR5 inhibitor. Nat Med 2005; 11: 1293-4.
[http://dx.doi.org/10.1038/nm1321] -34Williams R, Bokhari S, Silverstein P, Pinson D, Kumar A, Buch S. Nonhuman primate models of NeuroAIDS. J Neurovirol 2008; 14: 292-300.
[http://dx.doi.org/10.1080/13550280802074539] ]. However, this model has limitations, as it does not support HIV replication, as well as requiring sometimes prohibitive cost for primates whose accessibility is limited, especially with regard to the females. In addition, it faces disparities in host SIV and SHIV vulnerability since the primate colonies are outbred. Another animal model currently in use for testing of microbicidal candidates is the BLT (human Bone marrow, Liver, Thymus) mouse [35Wege AK, Melkus MW, Denton PW, Estes JD, Garcia JV. Functional and phenotypic characterization of the humanized BLT mouse model. Curr Top Microbiol Immunol 2008; 324: 149-65.
[http://dx.doi.org/10.1007/978-3-540-75647-7_10] -47Chateau ML, Denton PW, Swanson MD, McGowan I, Garcia JV. Rectal transmission of transmitted/founder HIV-1 is efficiently prevented by topical 1% tenofovir in BLT humanized mice. PLoS One 2013; 8: e60024.
[http://dx.doi.org/10.1371/journal.pone.0060024] ]. These humanized BLT mice are created by the implantation of human fetal liver and thymus tissues under the kidney capsule of an immunodeficient NOD scid gamma (NSG = NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ) mouse, followed three weeks later by the injection of autologous human fetal liver CD34+ cells (human hematopoietic stem cells, HSC). In the BLT mouse, a complete systemic reconstitution is achieved for all of the major human hematopoietic lineages, including T, B, monocyte/macrophage, dendritic, and natural killer cells. In addition, the developing T cell education occurs in the human thymic tissue. Fortuitously, for their use in the testing of microbicides, this widespread systemic and genital mucosal reconstitution with human lymphoid cells make female humanized BLT mice a useful tool to test both vaginal and rectal HIV transmission.
The short amphipathic helical peptide SWLRDIWDWICEVLSDFK called C5A, was originally identified by the Chisari lab as an active anti-hepatitis C virus (HCV) agent [48Cheng G, Montero A, Gastaminza P, et al. A virocidal amphipathic {alpha}-helical peptide that inhibits hepatitis C virus infection in vitro. Proc Natl Acad Sci USA 2008; 105: 3088-93.
[http://dx.doi.org/10.1073/pnas.0712380105] ]. The C5A amino acid sequence encompasses the region that permits the anchoring of the HCV nonstructural protein 5A (NS5A) into the ER membrane by an in-plane amphipathic alpha-helix [49Brass V, Bieck E, Montserret R, et al. An amino-terminal amphipathic alpha-helix mediates membrane association of the hepatitis C virus nonstructural protein 5A. J Biol Chem 2002; 277: 8130-9.
[http://dx.doi.org/10.1074/jbc.M111289200] , 50Penin F, Brass V, Appel N, et al. Structure and function of the membrane anchor domain of hepatitis C virus nonstructural protein 5A. J Biol Chem 2004; 279: 40835-43.
[http://dx.doi.org/10.1074/jbc.M404761200] ] (Fig. 1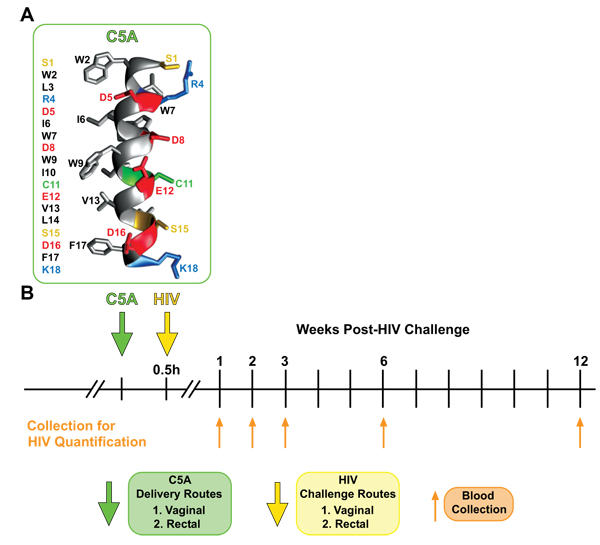 ). In collaboration with the Chisari lab, we demonstrated that C5A also exerts antiviral activities against HIV [51Bobardt MD, Cheng G, de Witte L, et al. Hepatitis C virus NS5A anchor peptide disrupts human immunodeficiency virus. Proc Natl Acad Sci USA 2008; 105: 5525-30.
). In collaboration with the Chisari lab, we demonstrated that C5A also exerts antiviral activities against HIV [51Bobardt MD, Cheng G, de Witte L, et al. Hepatitis C virus NS5A anchor peptide disrupts human immunodeficiency virus. Proc Natl Acad Sci USA 2008; 105: 5525-30.
[http://dx.doi.org/10.1073/pnas.0801388105] ]. We found that C5A inhibits a broad range of primary HIV isolates at an nM-µM peptide concentration range and blocks infection of the three major in vivo targets of HIV; CD4+ T-lymphocytes, macrophages and dendritic cells [51Bobardt MD, Cheng G, de Witte L, et al. Hepatitis C virus NS5A anchor peptide disrupts human immunodeficiency virus. Proc Natl Acad Sci USA 2008; 105: 5525-30.
[http://dx.doi.org/10.1073/pnas.0801388105] ]. Using an in vitro transwell chamber assay, which mimics HIV transmigration through primary human genital epithelial cells, we also demonstrated that C5A prevents the passage of HIV through this in vitro genital epithelial barrier [51Bobardt MD, Cheng G, de Witte L, et al. Hepatitis C virus NS5A anchor peptide disrupts human immunodeficiency virus. Proc Natl Acad Sci USA 2008; 105: 5525-30.
[http://dx.doi.org/10.1073/pnas.0801388105] ]. Moreover, we showed that C5A prevents HIV transfer from Langerhans and dendritic cells to CD4+ T-lymphocytes [51Bobardt MD, Cheng G, de Witte L, et al. Hepatitis C virus NS5A anchor peptide disrupts human immunodeficiency virus. Proc Natl Acad Sci USA 2008; 105: 5525-30.
[http://dx.doi.org/10.1073/pnas.0801388105] ]. Importantly, we obtained evidence for the antiviral mechanism of action of C5A. We demonstrated that C5A disrupts the membrane integrity of the HIV virion as well as the integrity of the conical capsid core that surrounds the viral genome, leading to non-infectious particles [51Bobardt MD, Cheng G, de Witte L, et al. Hepatitis C virus NS5A anchor peptide disrupts human immunodeficiency virus. Proc Natl Acad Sci USA 2008; 105: 5525-30.
[http://dx.doi.org/10.1073/pnas.0801388105] ]. Most importantly, although C5A destabilizes the viral membrane, we showed that it preserves the integrity of cellular membranes and is not toxic to cervical epithelial cells even when used at a concentration of 10- to 100-fold greater than that which blocks HIV infection [51Bobardt MD, Cheng G, de Witte L, et al. Hepatitis C virus NS5A anchor peptide disrupts human immunodeficiency virus. Proc Natl Acad Sci USA 2008; 105: 5525-30.
[http://dx.doi.org/10.1073/pnas.0801388105] ].
In this study, we tested the in vivo microbicidal efficacy of C5A against vaginal and rectal HIV challenges using the BLT mouse model. Additionally, we tested a panel of C5A variants with various degrees of anti-infectivity efficacy for their capacities to prevent vaginal and rectal HIV transmission. The main goal of this study was to investigate the microbicidal potency of C5A in vivo and to examine the molecular requirements of the structure and composition of C5A that determine its microbicidal activity. These data will be used to determine the most effective combination of C5A derivatives and well-characterized, small molecule ARVs with anti-HIV mechanisms of action distinct from that of C5A (i.e., elvitegravir, FTC or TDF), for formulation as an intravaginal ring nBP product.
2. MATERIAL AND METHODS
2.1. Creation of Humanized BLT Mice
Humanized BLT mice were created as described previously [36Denton PW, Estes JD, Sun Z, et al. Antiretroviral pre-exposure prophylaxis prevents vaginal transmission of HIV-1 in humanized BLT mice. PLoS Med 2008; 5: e16.
[http://dx.doi.org/10.1371/journal.pmed.0050016] -38Denton PW, Othieno F, Martinez-Torres F, et al. One percent tenofovir applied topically to humanized BLT mice and used according to the CAPRISA 004 experimental design demonstrates partial protection from vaginal HIV infection, validating the BLT model for evaluation of new microbicide candidates. J Virol 2011; 85: 7582-93.
[http://dx.doi.org/10.1128/JVI.00537-11] , 40Chateau ML, Denton PW, Swanson MD, McGowan I, Garcia JV. Rectal transmission of transmitted/founder HIV-1 is efficiently prevented by topical 1% tenofovir in BLT humanized mice. PLoS One 2013; 8: e60024.
[http://dx.doi.org/10.1371/journal.pone.0060024] , 42Wahl A, Victor Garcia J. The use of BLT humanized mice to investigate the immune reconstitution of the gastrointestinal tract. J Immunol Methods 2014; 410: 28-33.
[http://dx.doi.org/10.1016/j.jim.2014.06.009] , 44Melkus MW, Estes JD, Padgett-Thomas A, et al. Humanized mice mount specific adaptive and innate immune response to EBV and TSST-1. Nat Med 2006; 12: 1316-22.
[http://dx.doi.org/10.1038/nm1431] -47Chateau ML, Denton PW, Swanson MD, McGowan I, Garcia JV. Rectal transmission of transmitted/founder HIV-1 is efficiently prevented by topical 1% tenofovir in BLT humanized mice. PLoS One 2013; 8: e60024.
[http://dx.doi.org/10.1371/journal.pone.0060024] , 52Lan P, Tonomura N, Shimizu A, Wang S, Yang YG. Reconstitution of a functional human immune system in immunodeficient mice through combined human fetal thymus/liver and CD34_ cell transplantation. Blood 2006; 108: 487-92.
[http://dx.doi.org/10.1182/blood-2005-11-4388] -54Stoddart CA, Maidji E, Galkina SA, et al. Superior human leukocyte reconstitution and susceptibility to vaginal HIV transmission in humanized NOD-scid IL-2Rγ(-/-) (NSG) BLT mice. Virology 2011; 417: 154-60.
[http://dx.doi.org/10.1016/j.virol.2011.05.013] ], by implanting 1-mm3 pieces of human fetal liver and thymus tissues (Advanced Bioscience Resources) under the kidney capsule of 6- to 8-week-old female NSG mice (Jackson Laboratories) bred at The Scripps Research Institute (TSRI). Each cohort was produced with tissues from a single donor. CD34+ hematopoietic stem and progenitor cells (HSPC) were purified from autologous fetal liver tissue, isolated by magnetic bead selection for CD34+ cells (Miltenyi), phenotyped by flow cytometry [36Denton PW, Estes JD, Sun Z, et al. Antiretroviral pre-exposure prophylaxis prevents vaginal transmission of HIV-1 in humanized BLT mice. PLoS Med 2008; 5: e16.
[http://dx.doi.org/10.1371/journal.pmed.0050016] -38Denton PW, Othieno F, Martinez-Torres F, et al. One percent tenofovir applied topically to humanized BLT mice and used according to the CAPRISA 004 experimental design demonstrates partial protection from vaginal HIV infection, validating the BLT model for evaluation of new microbicide candidates. J Virol 2011; 85: 7582-93.
[http://dx.doi.org/10.1128/JVI.00537-11] , 40Chateau ML, Denton PW, Swanson MD, McGowan I, Garcia JV. Rectal transmission of transmitted/founder HIV-1 is efficiently prevented by topical 1% tenofovir in BLT humanized mice. PLoS One 2013; 8: e60024.
[http://dx.doi.org/10.1371/journal.pone.0060024] , 42Wahl A, Victor Garcia J. The use of BLT humanized mice to investigate the immune reconstitution of the gastrointestinal tract. J Immunol Methods 2014; 410: 28-33.
[http://dx.doi.org/10.1016/j.jim.2014.06.009] , 44Melkus MW, Estes JD, Padgett-Thomas A, et al. Humanized mice mount specific adaptive and innate immune response to EBV and TSST-1. Nat Med 2006; 12: 1316-22.
[http://dx.doi.org/10.1038/nm1431] -47Chateau ML, Denton PW, Swanson MD, McGowan I, Garcia JV. Rectal transmission of transmitted/founder HIV-1 is efficiently prevented by topical 1% tenofovir in BLT humanized mice. PLoS One 2013; 8: e60024.
[http://dx.doi.org/10.1371/journal.pone.0060024] , 52Lan P, Tonomura N, Shimizu A, Wang S, Yang YG. Reconstitution of a functional human immune system in immunodeficient mice through combined human fetal thymus/liver and CD34_ cell transplantation. Blood 2006; 108: 487-92.
[http://dx.doi.org/10.1182/blood-2005-11-4388] -54Stoddart CA, Maidji E, Galkina SA, et al. Superior human leukocyte reconstitution and susceptibility to vaginal HIV transmission in humanized NOD-scid IL-2Rγ(-/-) (NSG) BLT mice. Virology 2011; 417: 154-60.
[http://dx.doi.org/10.1016/j.virol.2011.05.013] ], and cryopreserved until injection (200,000-350,000 CD34+ cells) into mice 3 weeks after fetal liver and thymus tissue implantation. Human cell population reconstitution in peripheral blood was confirmed by flow cytometry as described previously [36Denton PW, Estes JD, Sun Z, et al. Antiretroviral pre-exposure prophylaxis prevents vaginal transmission of HIV-1 in humanized BLT mice. PLoS Med 2008; 5: e16.
[http://dx.doi.org/10.1371/journal.pmed.0050016] -38Denton PW, Othieno F, Martinez-Torres F, et al. One percent tenofovir applied topically to humanized BLT mice and used according to the CAPRISA 004 experimental design demonstrates partial protection from vaginal HIV infection, validating the BLT model for evaluation of new microbicide candidates. J Virol 2011; 85: 7582-93.
[http://dx.doi.org/10.1128/JVI.00537-11] , 40Chateau ML, Denton PW, Swanson MD, McGowan I, Garcia JV. Rectal transmission of transmitted/founder HIV-1 is efficiently prevented by topical 1% tenofovir in BLT humanized mice. PLoS One 2013; 8: e60024.
[http://dx.doi.org/10.1371/journal.pone.0060024] , 42Wahl A, Victor Garcia J. The use of BLT humanized mice to investigate the immune reconstitution of the gastrointestinal tract. J Immunol Methods 2014; 410: 28-33.
[http://dx.doi.org/10.1016/j.jim.2014.06.009] , 44Melkus MW, Estes JD, Padgett-Thomas A, et al. Humanized mice mount specific adaptive and innate immune response to EBV and TSST-1. Nat Med 2006; 12: 1316-22.
[http://dx.doi.org/10.1038/nm1431] -47Chateau ML, Denton PW, Swanson MD, McGowan I, Garcia JV. Rectal transmission of transmitted/founder HIV-1 is efficiently prevented by topical 1% tenofovir in BLT humanized mice. PLoS One 2013; 8: e60024.
[http://dx.doi.org/10.1371/journal.pone.0060024] , 52Lan P, Tonomura N, Shimizu A, Wang S, Yang YG. Reconstitution of a functional human immune system in immunodeficient mice through combined human fetal thymus/liver and CD34_ cell transplantation. Blood 2006; 108: 487-92.
[http://dx.doi.org/10.1182/blood-2005-11-4388] -54Stoddart CA, Maidji E, Galkina SA, et al. Superior human leukocyte reconstitution and susceptibility to vaginal HIV transmission in humanized NOD-scid IL-2Rγ(-/-) (NSG) BLT mice. Virology 2011; 417: 154-60.
[http://dx.doi.org/10.1016/j.virol.2011.05.013] ]. Briefly, the degree of humanization of the BLT mice was evaluated at 20 weeks of age (10 weeks post-CD34+ HSPC injection) by analysis of percentages of human CD45+ cells and human CD45+ CD4+ CD3+ cells in peripheral blood by FACS. Mice with <65% human CD45+ cells and <70% human CD45+ CD4+ CD3+ cells were not used for HIV exposure experiments. Mice were kept at the Department of Animal Resources at TSRI in accordance with protocols approved by the TSRI Institutional Animal Care and Use Committee.
 |
Fig. (1) Experimental strategy for the HIV genital transmission model in humanized mice. A. Presented are the amino acid sequence and three-dimensional structure of the short helical peptide SWLRDIWDWICEVLSDFK C5A deduced from the nuclear magnetic resonance structure of the NS5A N-terminal membrane anchor. The image was created from structure coordinates using VMD (http://www.ks.uiuc.edu/Research/vmd/). B. Experimental timeline for the “humanization” of mice including the engraftment of human fetal tissues, the inoculation of autologous human CD34+ liver cells as well as the topical application of C5A prior to vaginal and rectal HIV challenges, and the viral load quantification in blood collected at the indicated time points. |
2.2. Vaginal and Rectal HIV Infection Studies in Humanized BLT Mice
The parent and modified C5A peptides were obtained from GenScript, and their amino acid sequences are given in Fig. (1A ) and Table 1. Solutions of C5A peptides were prepared in phosphate buffered saline (PBS). After verifying the reconstitution of mice with human cells, a dose ranging study was conducted to determine the EC50 of C5A for preventing vaginal and rectal HIV infection. Stocks of HIV JR-CSF were prepared as previously described [46Denton PW, Othieno F, Martinez-Torres F, et al. One percent tenofovir applied topically to humanized BLT mice and used according to the CAPRISA 004 experimental design demonstrates partial protection from vaginal HIV infection, validating the BLT model for evaluation of new microbicide candidates. J Virol 2011; 85: 7582-93.
) and Table 1. Solutions of C5A peptides were prepared in phosphate buffered saline (PBS). After verifying the reconstitution of mice with human cells, a dose ranging study was conducted to determine the EC50 of C5A for preventing vaginal and rectal HIV infection. Stocks of HIV JR-CSF were prepared as previously described [46Denton PW, Othieno F, Martinez-Torres F, et al. One percent tenofovir applied topically to humanized BLT mice and used according to the CAPRISA 004 experimental design demonstrates partial protection from vaginal HIV infection, validating the BLT model for evaluation of new microbicide candidates. J Virol 2011; 85: 7582-93.
[http://dx.doi.org/10.1128/JVI.00537-11] , 47Chateau ML, Denton PW, Swanson MD, McGowan I, Garcia JV. Rectal transmission of transmitted/founder HIV-1 is efficiently prevented by topical 1% tenofovir in BLT humanized mice. PLoS One 2013; 8: e60024.
[http://dx.doi.org/10.1371/journal.pone.0060024] ] and standardized by p24 ELISA. Prior to inoculation, mice were anesthetized with isoflurane. Aliquots of C5A in PBS (5 µL) were administered vaginally or rectally in decreasing concentrations (200, 100, 50, 25, 12.5 and 6.25 µM; n=10 mice per concentration). Fifteen minutes post-C5A topical administration, mice were challenged (vaginal challenge for vaginal C5A administration, rectal challenge for C5A rectal administration) with HIV. Atraumatic vaginal and rectal HIV inoculations were conducted as previously described [46Denton PW, Othieno F, Martinez-Torres F, et al. One percent tenofovir applied topically to humanized BLT mice and used according to the CAPRISA 004 experimental design demonstrates partial protection from vaginal HIV infection, validating the BLT model for evaluation of new microbicide candidates. J Virol 2011; 85: 7582-93.
[http://dx.doi.org/10.1128/JVI.00537-11] , 47Chateau ML, Denton PW, Swanson MD, McGowan I, Garcia JV. Rectal transmission of transmitted/founder HIV-1 is efficiently prevented by topical 1% tenofovir in BLT humanized mice. PLoS One 2013; 8: e60024.
[http://dx.doi.org/10.1371/journal.pone.0060024] ] using a total volume of 5 µL (200 ng of p24 - 20,000 of 50% tissue culture infective dose (TCID50%) (Fig. 1B ). Infection was quantified by measuring levels of viral RNA in peripheral blood at weeks 1, 2, 3, 6 and 12. Infection studies comparing the modified C5A peptides to the C5A parent were conducted using an identical procedure, with vaginal or rectal application of a single 100 µM peptide dose (n=10 mice per peptide group) 15 min prior to vaginal or rectal HIV challenge, respectively. Note that upon viral genome sequencing, viruses that replicate in the peripheral blood of mice did not differ from the inoculated JR-CSF (data not shown).
). Infection was quantified by measuring levels of viral RNA in peripheral blood at weeks 1, 2, 3, 6 and 12. Infection studies comparing the modified C5A peptides to the C5A parent were conducted using an identical procedure, with vaginal or rectal application of a single 100 µM peptide dose (n=10 mice per peptide group) 15 min prior to vaginal or rectal HIV challenge, respectively. Note that upon viral genome sequencing, viruses that replicate in the peripheral blood of mice did not differ from the inoculated JR-CSF (data not shown).
2.3. Viral Load Quantification in Humanized BLT Mice
Infection of BLT mice was analyzed by quantifying HIV RNA levels in peripheral blood (plasma) using one-step reverse transcriptase quantitative real-time PCR (ABI custom TaqMan Assays-by-Design) according to the manufacturer’s instructions. Primers were 5-CATGTTTTCAGCATTATCAGAAGGA-3 and 5-TGCTTGATGT CCCCCCACT-3, and MGB-probe 5-FAM-CCACCCCACAAGATTTAAACACCATGCTAA-Q-3, where FAM is 6-carboxyfluorescein [36Denton PW, Estes JD, Sun Z, et al. Antiretroviral pre-exposure prophylaxis prevents vaginal transmission of HIV-1 in humanized BLT mice. PLoS Med 2008; 5: e16.
[http://dx.doi.org/10.1371/journal.pmed.0050016] -38Denton PW, Othieno F, Martinez-Torres F, et al. One percent tenofovir applied topically to humanized BLT mice and used according to the CAPRISA 004 experimental design demonstrates partial protection from vaginal HIV infection, validating the BLT model for evaluation of new microbicide candidates. J Virol 2011; 85: 7582-93.
[http://dx.doi.org/10.1128/JVI.00537-11] , 40Chateau ML, Denton PW, Swanson MD, McGowan I, Garcia JV. Rectal transmission of transmitted/founder HIV-1 is efficiently prevented by topical 1% tenofovir in BLT humanized mice. PLoS One 2013; 8: e60024.
[http://dx.doi.org/10.1371/journal.pone.0060024] , 42Wahl A, Victor Garcia J. The use of BLT humanized mice to investigate the immune reconstitution of the gastrointestinal tract. J Immunol Methods 2014; 410: 28-33.
[http://dx.doi.org/10.1016/j.jim.2014.06.009] , 44Melkus MW, Estes JD, Padgett-Thomas A, et al. Humanized mice mount specific adaptive and innate immune response to EBV and TSST-1. Nat Med 2006; 12: 1316-22.
[http://dx.doi.org/10.1038/nm1431] -47Chateau ML, Denton PW, Swanson MD, McGowan I, Garcia JV. Rectal transmission of transmitted/founder HIV-1 is efficiently prevented by topical 1% tenofovir in BLT humanized mice. PLoS One 2013; 8: e60024.
[http://dx.doi.org/10.1371/journal.pone.0060024] , 52Lan P, Tonomura N, Shimizu A, Wang S, Yang YG. Reconstitution of a functional human immune system in immunodeficient mice through combined human fetal thymus/liver and CD34_ cell transplantation. Blood 2006; 108: 487-92.
[http://dx.doi.org/10.1182/blood-2005-11-4388] -54Stoddart CA, Maidji E, Galkina SA, et al. Superior human leukocyte reconstitution and susceptibility to vaginal HIV transmission in humanized NOD-scid IL-2Rγ(-/-) (NSG) BLT mice. Virology 2011; 417: 154-60.
[http://dx.doi.org/10.1016/j.virol.2011.05.013] ]. The assay sensitivity was of 300-500 400 RNA copies per mL.
2.4. Data Analysis
Data were analyzed using GraphPad Prism (version 7.00, GraphPad Software, Inc., La Jolla, CA). Analytic simulations of dose-response curves using the median-effect principle and mass-action law, and its combination index theorem [55Chou TC. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol Rev 2006; 58: 621-81.
[http://dx.doi.org/10.1124/pr.58.3.10] ] were carried out using CompuSyn [56Chou TC, Martin N. CompuSyn for drug combinations: PC software and user’s guide: A computer program for quantitation of synergism and antagonism in drug combinations, and the determination of IC50 and ED50 and LD50 values 2005.].
3. RESULTS
3.1. Dose-ranging Study to Evaluate C5A Efficacy Against Vaginal and Rectal HIV Challenges in BLT Mice
The amounts of topically applied C5A required to block HIV transmission in BLT mice challenged either vaginally or rectally were evaluated in a dose-ranging infection study. Vaginally applied C5A offered no protection against a vaginal HIV challenge at 6.25 and 12.5 µM, 30% protection at 25 µM, 80% protection at 50 µM and 100% protection at 100 and 200 µM (Table 2). Similar protection results were obtained for the rectal challenge of HIV: no protection at 6.25 and 12.5 µM, 50% protection at 25 µM, 80% protection at 50 µM and 100% protection at 100 and 200 µM (Table 2 and Appendix. A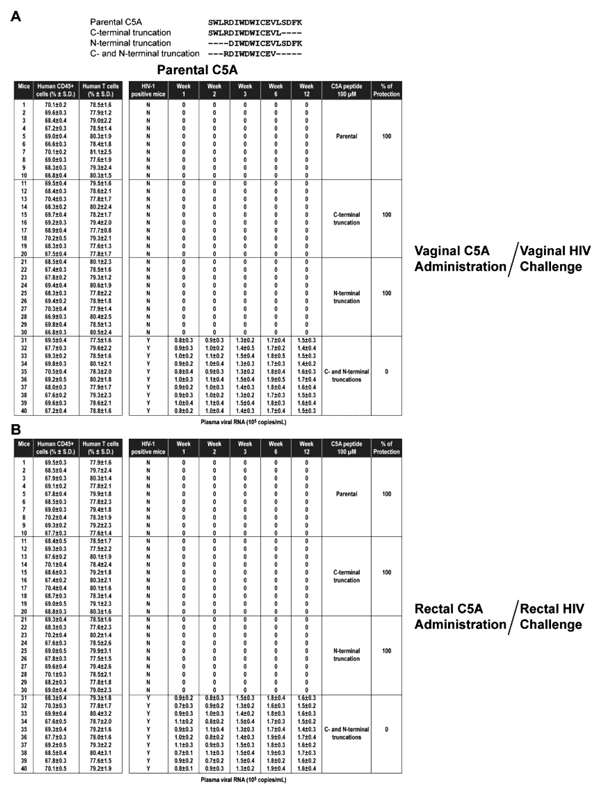 ). The absence of HIV RNA in blood correlated well with the absence of HIV DNA in tissues including spleen, lymph node, thymic organoid, liver, lung and female reproductive tract (data not shown). Dose-response curves for vaginal and rectal HIV prevention efficacy by C5A are shown in Fig. (2
). The absence of HIV RNA in blood correlated well with the absence of HIV DNA in tissues including spleen, lymph node, thymic organoid, liver, lung and female reproductive tract (data not shown). Dose-response curves for vaginal and rectal HIV prevention efficacy by C5A are shown in Fig. (2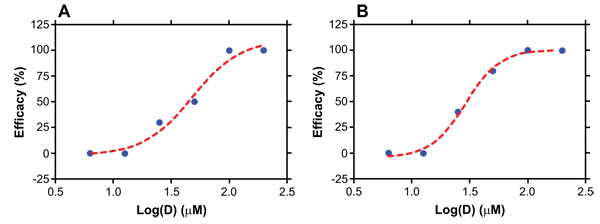 ), along with fits of the data to a sigmoidal dose-response (variable slope) model. From the model, EC50 values of 32.5 and 25.7 μM were calculated for vaginal and rectal dosing, respectively.
), along with fits of the data to a sigmoidal dose-response (variable slope) model. From the model, EC50 values of 32.5 and 25.7 μM were calculated for vaginal and rectal dosing, respectively.
3.2. Effect of Amino Acid Sequence Modifications on C5A Microbicidal Properties
The protection efficacy of C5A was compared to efficacy of modified C5A peptides with C-terminal (C5A-C), N-terminal (C5A-N), or C- and N-terminal truncations (C5A-CN). For 100 µM C5A (18 amino acid length), C5A-C (14 amino acid length), or C5A-N (14 amino acid length), 100% protective efficacy was maintained against a vaginal or a rectal HIV challenge (Table 3 and Appendix. B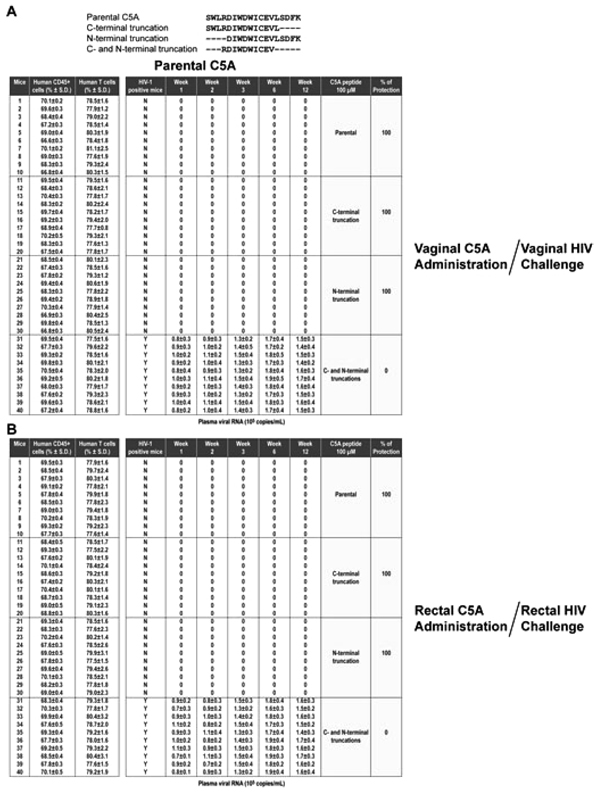 ). In sharp contrast, C5A-CN (10 amino acid length) with both ends truncated offered no protection (Table 3). Both the non-amphipathic (C5A-NA) and hydrophobicity-scrambled (C5A-HS) variants show no protection against vaginal or rectal challenge by HIV (Table 3 and Appendix. C
). In sharp contrast, C5A-CN (10 amino acid length) with both ends truncated offered no protection (Table 3). Both the non-amphipathic (C5A-NA) and hydrophobicity-scrambled (C5A-HS) variants show no protection against vaginal or rectal challenge by HIV (Table 3 and Appendix. C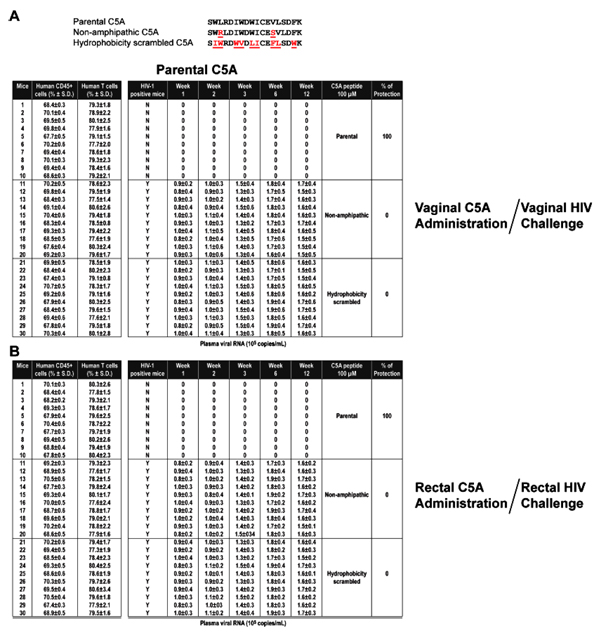 ), suggesting that the amphipathic and hydrophobic nature of C5A is required for its ability to block vaginal and rectal HIV transmission in humanized BLT mice.
), suggesting that the amphipathic and hydrophobic nature of C5A is required for its ability to block vaginal and rectal HIV transmission in humanized BLT mice.
 |
Fig. (2) Calculation of EC50 of C5A providing protection against (A) vaginal and (B) rectal HIV challenges in humanized BLT mice. |
4. DISCUSSION AND CONCLUSION
In the absence of a safe and effective vaccine, there is an imperative demand for effective agents to reduce the 7,000 new HIV infections occurring every day. An attractive possibility is to identify the best drugs and drug combinations, delivered by optimal nBP methods such Intravaginal Rings (IVRs) for vaginal delivery and gels or enemas for rectal protection. We previously reported that C5A, a short, amphipathic, 18 amino acid (SWLRDIWDWICEVLSDFK) helical peptide, inhibits in vitro infectivity of a broad range of primary HIV isolates in various primary target cells [51Bobardt MD, Cheng G, de Witte L, et al. Hepatitis C virus NS5A anchor peptide disrupts human immunodeficiency virus. Proc Natl Acad Sci USA 2008; 105: 5525-30.
[http://dx.doi.org/10.1073/pnas.0801388105] ]. In this study, we evaluated the microbicidal efficacy of C5A in vivo in a humanized mouse model, and investigated the structural requirements of C5A to block HIV transmission in vivo. These studies show that parental C5A efficiently inhibits both vaginal and rectal HIV transmission in humanized mice. This is in accordance with a previous study demonstrating that topical vaginal administration of 200 µM C5A in PBS confers 100% protection against a vaginal challenge of HIV in humanized mice [38Denton PW, Othieno F, Martinez-Torres F, et al. One percent tenofovir applied topically to humanized BLT mice and used according to the CAPRISA 004 experimental design demonstrates partial protection from vaginal HIV infection, validating the BLT model for evaluation of new microbicide candidates. J Virol 2011; 85: 7582-93.
[http://dx.doi.org/10.1128/JVI.00537-11] ]. This is also in accordance with our previous study that showed that C5A maintains its antiviral activities in genital fluids including seminal plasma, cervical and vaginal fluids [57de Witte L, Bobardt MD, Chatterji U, et al. HSV neutralization by the microbicidal candidate C5A. PLoS One 2011; 6: e18917.
[http://dx.doi.org/10.1371/journal.pone.0018917] ]. Moreover, this is in agreement with our recent study that showed that a vaginal application of C5A protects 89% of macaques from a simian-human immunodeficiency virus (SHIV-162P3) challenge [58Veazey RS, Chatterji U, Bobardt M, et al. C5A protects macaques from vaginal simian-human immunodeficiency virus challenge. Antimicrob Agents Chemother 2015; 60: 693-8.
[http://dx.doi.org/10.1128/AAC.01925-15] ]. Although the vaginal pH varies between species – 3.5 to 4.5 for humans and 6 to 7 for mice and macaques is 6 to 7 [59Zeitlin L, Hoen TE, Achilles SL, et al. Tests of Buffergel for contraception and prevention of sexually transmitted diseases in animal models. Sex Transm Dis 2001; 28: 417-23.
[http://dx.doi.org/10.1097/00007435-200107000-00010] , 60Daggett GJ Jr, Zhao C, Connor-Stroud F, et al. Comparison of the vaginal environment in rhesus and cynomolgus macaques pre- and post-lactobacillus colonization. J Med Primatol 2017; 46: 232-8.
[http://dx.doi.org/10.1111/jmp.12264] ] - we previously reported that C5A keeps is antiviral activity between pH 5 and 7, but loses its activity at pH 8 [51Bobardt MD, Cheng G, de Witte L, et al. Hepatitis C virus NS5A anchor peptide disrupts human immunodeficiency virus. Proc Natl Acad Sci USA 2008; 105: 5525-30.
[http://dx.doi.org/10.1073/pnas.0801388105] ]. For the humanized mouse studies reported here, 50% effective concentrations (EC50) of C5A calculated using the traditional sigmodal dose-response model were 32.5 and 25.7 µM, respectively, for vaginal and rectal HIV challenge (Fig. 2 ). A major advantage of the humanized mouse model is the ability to create large groups of animals from tissues from a single donor. We routinely generate 60-80 humanized mice per liver/thymus tissue. Another advantage of this model is the capacity of using high numbers of animals per group/treatment (n = 10) to attain a satisfactory statistical power. This further confirms that the BLT mouse model is an ideal tool to screen microbicidal candidates.
). A major advantage of the humanized mouse model is the ability to create large groups of animals from tissues from a single donor. We routinely generate 60-80 humanized mice per liver/thymus tissue. Another advantage of this model is the capacity of using high numbers of animals per group/treatment (n = 10) to attain a satisfactory statistical power. This further confirms that the BLT mouse model is an ideal tool to screen microbicidal candidates.
Applying the median-effect model to the humanized mouse dose-ranging efficacy datasets allowed EC values in the 50-97% protection range to be calculated (Fig. 3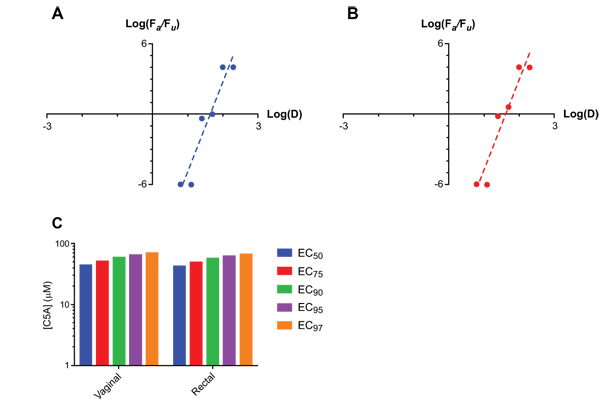 ). The slope, m, of the linear fit to the log-log median-effects plots Figs. (3A
). The slope, m, of the linear fit to the log-log median-effects plots Figs. (3A and 3B
and 3B ) is analogous to the Hill coefficient and provides a quantitative estimation of the sigmoidicity of the dose-effect curve. Slope parameters (m) for C5A of 7.6 ± 1.2 and 7.7 ± 1.2 were calculated for the vaginal and rectal compartments, respectively. Fig. (3
) is analogous to the Hill coefficient and provides a quantitative estimation of the sigmoidicity of the dose-effect curve. Slope parameters (m) for C5A of 7.6 ± 1.2 and 7.7 ± 1.2 were calculated for the vaginal and rectal compartments, respectively. Fig. (3 ) summarizes the results from this expanded analsyis, where Fa represents the fraction affected and Fu the fraction unaffected. The high regression coefficients (r > 0.95) support the applicability of the model. Shen et al. used an ex vivo HIV model to show that ARV agents has a characteristic slope, ranging from 1 for nucleoside reverse transcriptase inhibitors (NRTIs) to 1.8-4.5 protease inhibitors (PIs) [61Shen L, Peterson S, Sedaghat AR, et al. Dose-response curve slope sets class-specific limits on inhibitory potential of anti-HIV drugs. Nat Med 2008; 14: 762-6.
) summarizes the results from this expanded analsyis, where Fa represents the fraction affected and Fu the fraction unaffected. The high regression coefficients (r > 0.95) support the applicability of the model. Shen et al. used an ex vivo HIV model to show that ARV agents has a characteristic slope, ranging from 1 for nucleoside reverse transcriptase inhibitors (NRTIs) to 1.8-4.5 protease inhibitors (PIs) [61Shen L, Peterson S, Sedaghat AR, et al. Dose-response curve slope sets class-specific limits on inhibitory potential of anti-HIV drugs. Nat Med 2008; 14: 762-6.
[http://dx.doi.org/10.1038/nm1777] ]. The much higher slopes (m) of the C5A dose-response curves (7.6 and 7.7 for vaginal and rectal dosing, respectively) observed in vivo suggest that this microbicide has significant potential as an efficatious PrEP agent, as illustrated in Fig. (3C ). To our knowledge, this is the first demonstration that the humanized BLT mouse model allows the determination of ECs of a drug against vaginal or rectal HIV transmission.
). To our knowledge, this is the first demonstration that the humanized BLT mouse model allows the determination of ECs of a drug against vaginal or rectal HIV transmission.
We found that the genital administration of 25 or 50 µM of C5A provides only a partial protection. We believe that these concentrations of the virucidal peptide in the genital compartment do not neutralize (rupture) 100% of incoming viruses, allowing some infectious particles to cross the genital epithelium and to reach the peripheral blood where CD4+ T lymphocytes will be infected and will support robust HIV replication.
The studies with modified C5A-C and C5A-N demonstrate that the removal of four amino acids at either end of C5A does not alter the microbicidal efficacy of the peptide. In sharp contrast, the simultaneous removal of four amino acids at each end (C5A-CN) abolishes the microbicidal capabilities of C5A, suggesting that the peptide requires a sufficient length to effectively block both vaginal and rectal HIV transmissions.
The lack of protection observed for C5A-NA demonstrates that the amphipathic structural nature of C5A is critical for its microbicidal properties. Similarly, C5A-HS offered no protection vaginally or rectally, indicating that the hydrophobic face of C5A (or NS5A), thought to mediate anchoring into the ER membrane, is also critical for the ability of the peptide to inhibit HIV infection.
Following these studies with unformulated C5A solutions applied topically, the next goal is to formulate C5A, alone and in combination with other antiretroviral agents, into murine intravaginal rings to evaluate sustained topical delivery approaches for protection against vaginal and rectal HIV challenges. We plan on using a combination of drugs with distinct mechanisms of action. Remarkably, C5A possesses a unique anti-HIV mechanism of action. We demonstrated that C5A disrupts the stability of the membrane of HIV virions as well as that of the capsid core, surrounding the viral genome, resulting into non-infectious particles [51Bobardt MD, Cheng G, de Witte L, et al. Hepatitis C virus NS5A anchor peptide disrupts human immunodeficiency virus. Proc Natl Acad Sci USA 2008; 105: 5525-30.
[http://dx.doi.org/10.1073/pnas.0801388105] ]. Our working hypothesis is that anchoring C5A molecules via their hydrophobic surface into the HIV membrane, destabilizes and ruptures the integrity of the viral membrane. This means that C5A theoretically would act in the lumen (vaginal or rectal), much like the traditional microbicides. This mode of action would be highly complementary to small-molecule ARV PrEP candidates (e.g., TDF and FTC) as these inhibit viral replication in immune cells, presumably in tissues (vaginal or rectal), a different anatomic compartment. Importantly, C5A does not alter the cellular membranes even when used at a concentration of 10- to 100-fold greater than that which blocks HIV infection [51Bobardt MD, Cheng G, de Witte L, et al. Hepatitis C virus NS5A anchor peptide disrupts human immunodeficiency virus. Proc Natl Acad Sci USA 2008; 105: 5525-30.
[http://dx.doi.org/10.1073/pnas.0801388105] ]. This is in accordance with our recent study that demonstrated that a vaginal application of C5A protects 89% of macaques from a simian-human immunodeficiency virus (SHIV-162P3) challenge and that no signs of lesions or inflammation were observed in animals vaginally treated with repeated C5A applications [57de Witte L, Bobardt MD, Chatterji U, et al. HSV neutralization by the microbicidal candidate C5A. PLoS One 2011; 6: e18917.
[http://dx.doi.org/10.1371/journal.pone.0018917] ]. With its noncellular cytotoxic activity and rare mechanism of action, C5A represents an attractive microbicidal candidate to be combined with other drugs, including ARVs, with different antiviral mechanisms of action.
An additional attractive property that C5A offers is that it also efficiently inhibits HSV infection [58Veazey RS, Chatterji U, Bobardt M, et al. C5A protects macaques from vaginal simian-human immunodeficiency virus challenge. Antimicrob Agents Chemother 2015; 60: 693-8.
[http://dx.doi.org/10.1128/AAC.01925-15] ], potentially acting as a single-agent multipurpose protection technology (MPT). Previous studies have shown that C5A inhibits HSV infection of Vero cells, human dendritic cells and primary genital epithelial cells in vitro as well as human epidermis (epidermal sheets) ex vivo, and that it inhibits acyclovir- and ganciclovir-resistant HSV isolates [58Veazey RS, Chatterji U, Bobardt M, et al. C5A protects macaques from vaginal simian-human immunodeficiency virus challenge. Antimicrob Agents Chemother 2015; 60: 693-8.
[http://dx.doi.org/10.1128/AAC.01925-15] ]. Remarkably, in an in vitro transwell assay, C5A prevented HIV transmigration by preserving the integrity of the genital epithelium that is normally severely compromised by HSV pre-infection [58Veazey RS, Chatterji U, Bobardt M, et al. C5A protects macaques from vaginal simian-human immunodeficiency virus challenge. Antimicrob Agents Chemother 2015; 60: 693-8.
[http://dx.doi.org/10.1128/AAC.01925-15] ]. Because genital herpes is a major risk factor in acquiring HIV infection, C5A represents a multipurpose microbicide candidate, which may interfere with HIV transmission via a dual mechanism of action – neutralizing both HSV and HIV.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Mice were kept at the Department of Animal Resources at TSRI in accordance with protocols approved by the TSRI Institutional Animal Care and Use Committee.
HUMAN AND ANIMAL RIGHTS
The reported experiments are in accordance with the standards of The US Public Health Service's “Policy on Humane Care and Use of Laboratory Animals,” and “Guide for the Care and Use of Laboratory Animals.”
CONSENT FOR PUBLICATION
Not applicable.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGMENTS
We thank Dr. I. Chen for the primary JR-CSF HIV strain obtained through the NIH AIDS Research and Reference Reagent Program). We thank Dr. F. Chisari for the initial discovery of C5A and for his supportive collaboration over the years. We greatly thank Drs. B. Torbett and C. Stoddart for helpful discussion and advice for the development of the humanized BLT mouse model at The Scripps Research Institute. We thank Karen J. Clingerman for her initial assistance with the surgeries. We thank Perrin Larton from Advanced Bioscience Resources from providing us with fetal tissues. We thank Dr. F. Penin for the three-dimensional C5A structure presented in Fig. (1 ). We acknowledge financial support from the U.S. Public Health Service grants no. AI087470, AI079782 and AI076005 (P.A.G.). This is publication no. 29496 from the Department of Immunology & Microbiology, The Scripps Research Institute, La Jolla, CA.
). We acknowledge financial support from the U.S. Public Health Service grants no. AI087470, AI079782 and AI076005 (P.A.G.). This is publication no. 29496 from the Department of Immunology & Microbiology, The Scripps Research Institute, La Jolla, CA.
APPENDIX
 |
APPENDIX B The removal of both ends of C5A abrogates its capacity to inhibit vaginal and rectal HIV transmission in humanized mice.Same as Appendix.(A  ) except that indicated on the right are the amino acid sequences of the C5A peptides (100 µM) applied vaginally (A) or rectally (B) 15 min prior to a vaginal (A) and rectal (B) HIV (JR-CSF) challenge. ) except that indicated on the right are the amino acid sequences of the C5A peptides (100 µM) applied vaginally (A) or rectally (B) 15 min prior to a vaginal (A) and rectal (B) HIV (JR-CSF) challenge.
|
 |
APPENDIX C The amphipathic and hydrophobic nature of C5A controls the ability of the short peptide to prevent vaginal and rectal HIV transmission in humanized mice. Same as Appendix.(A  ) except that indicated on the right are the amino acid sequences of the C5A peptides (100 µM) applied vaginally (A) or rectally (B) 15 min prior to a vaginal (A) and rectal (B) HIV (JR-CSF) challenge. ) except that indicated on the right are the amino acid sequences of the C5A peptides (100 µM) applied vaginally (A) or rectally (B) 15 min prior to a vaginal (A) and rectal (B) HIV (JR-CSF) challenge.
|
REFERENCES
| [1] | 2010.WHO-UNAIDS Report on the Global AIDS Epidemic http://www.unaids.org/ globalreport/global_report.htm |
| [2] | Cohen MS, Gay C, Kashuba AD, Blower S, Paxton L. Narrative review: Antiretroviral therapy to prevent the sexual transmission of HIV-1. Ann Intern Med 2007; 146: 591-601. [http://dx.doi.org/10.7326/0003-4819-146-8-200704170-00010] |
| [3] | Derdelinckx I, Wainberg MA, Lange JM, Hill A, Halima Y, Boucher CA. Criteria for drugs used in pre-exposure prophylaxis trials against HIV infection. PLoS Med 2006; 3: e454. [http://dx.doi.org/10.1371/journal.pmed.0030454] |
| [4] | Cohen MS, Gay CL. Treatment to prevent transmission of HIV-1. Clin Infect Dis 2010; 50(Suppl. 3): S85-95. [http://dx.doi.org/10.1086/651478] |
| [5] | Grant RM, Buchbinder S, Cates W Jr, et al. Promote HIV chemoprophylaxis research, don’t prevent it. Science 2005; 309: 2170-1. [http://dx.doi.org/10.1126/science.1116204] |
| [6] | Landovitz RJ. Recent efforts in biomedical prevention of HIV. Top HIV Med 2007; 15(3): 99-103. |
| [7] | Liu AY, Grant RM, Buchbinder SP. Preexposure prophylaxis for HIV: Unproven promise and potential pitfalls. JAMA 2006; 296: 863-5. [http://dx.doi.org/10.1001/jama.296.7.863] |
| [8] | Mascolini M, Kort R, Gilden D. XVII International AIDS Conference: From Evidence to Action - Clinical and biomedical prevention science. J Int AIDS Soc 2009; 12(Suppl. 1): S4. [http://dx.doi.org/10.1186/1758-2652-12-S1-S4] |
| [9] | Paxton LA, Hope T, Jaffe HW. Pre-exposure prophylaxis for HIV infection: What if it works? Lancet 2007; 370: 89-93. [http://dx.doi.org/10.1016/S0140-6736(07)61053-8] |
| [10] | Youle M, Wainberg MA. Could chemoprophylaxis be used as an HIV prevention strategy while we wait for an effective vaccine? AIDS 2003; 17: 937-8. [http://dx.doi.org/10.1097/00002030-200304110-00027] |
| [11] | Cutler B, Justman J. Vaginal microbicides and the prevention of HIV transmission. Lancet Infect Dis 2008; 8: 685-97. [http://dx.doi.org/10.1016/S1473-3099(08)70254-8] |
| [12] | McGowan I. Microbicides for HIV prevention: reality or hope? Curr Opin Infect Dis 2010; 23: 26-31. [http://dx.doi.org/10.1097/QCO.0b013e328334fe70] |
| [13] | Baeten J, Grant R. Use of antiretrovirals for HIV prevention: what do we know and what don’t we know? Curr HIV/AIDS Rep 2013; 10: 142-51. [http://dx.doi.org/10.1007/s11904-013-0157-9] |
| [14] | van der Straten A, Stadler J, Luecke E, et al. Perspectives on use of oral and vaginal antiretrovirals for HIV prevention: The VOICE-C qualitative study in Johannesburg, South Africa. J Int AIDS Soc 2014; 17(3)(Suppl. 2): 19146. [http://dx.doi.org/10.7448/IAS.17.3.19146] |
| [15] | Turpin JA. Topical microbicides to prevent the transmission of HIV: Formulation gaps and challenges. Drug Deliv Transl Res 2011; 1: 194-200. [http://dx.doi.org/10.1007/s13346-011-0034-2] |
| [16] | Abdool Karim Q, Abdool Karim SS, Frohlich JA, et al. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science 2010; 329: 1168-74. [http://dx.doi.org/10.1126/science.1193748] |
| [17] | Grant RM, Lama JR, Anderson PL, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med 2010; 363: 2587-99. [http://dx.doi.org/10.1056/NEJMoa1011205] |
| [18] | Baeten JM, Donnell D, Ndase P, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med 2012; 367: 399-410. [http://dx.doi.org/10.1056/NEJMoa1108524] |
| [19] | Thigpen MC, Kebaabetswe PM, Paxton LA, et al. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med 2012; 367: 423-34. [http://dx.doi.org/10.1056/NEJMoa1110711] |
| [20] | Van Damme L, Corneli A, Ahmed K, et al. Preexposure prophylaxis for HIV infection among African women. N Engl J Med 2012; 367: 411-22. [http://dx.doi.org/10.1056/NEJMoa1202614] |
| [21] | Choopanya K, Martin M, Suntharasamai P, et al. Antiretroviral prophylaxis for HIV infection in injecting drug users in bangkok, thailand (the Bangkok Tenofovir Study): A randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2013; 381: 2083-90. [http://dx.doi.org/10.1016/S0140-6736(13)61127-7] |
| [22] | Molina JM, Capitant C, Spire B, et al. On-demand preexposure prophylaxis in men at high risk for HIV-1 infection. N Engl J Med 2015; 373: 2237-46. [http://dx.doi.org/10.1056/NEJMoa1506273] |
| [23] | Marcus JL, Hurley LB, Hare CB, et al. Preexposure prophylaxis for HIV prevention in a large integrated health care system: Adherence, renal safety, and discontinuation. J Acquir Immune Defic Syndr 2016; 73: 540-6. [http://dx.doi.org/10.1097/QAI.0000000000001129] |
| [24] | McCormack S, Dunn DT, Desai M, et al. Pre-exposure prophylaxis to prevent the acquisition of HIV-1 infection (PROUD): Effectiveness results from the pilot phase of a pragmatic open-label randomised Trial. Lancet 2016; 387: 53-60. [http://dx.doi.org/10.1016/S0140-6736(15)00056-2] |
| [25] | Delany-Moretlwe S, Smith E, Myer L, et al. FACTS 001: Characteristics of participants enrolled in a phase III randomised controlled trial of tenofovir gel for prevention of HIV-1 and HSV-2. AIDS Res Hum Retroviruses 2014; 30: A281-2. [http://dx.doi.org/10.1089/aid.2014.5637.abstract] |
| [26] | Marrazzo JM, Ramjee G, Richardson BA, Gomez K, Mgodi N, Nair G, et al. Tenofovir-based preexposure prophylaxis for HIV infection among african women. Obstet Gynecol Surv 2015; 70: 444-6. [http://dx.doi.org/10.1097/01.ogx.0000466878.37011.6f] |
| [27] | Baeten JM, Palanee-Phillips T, Brown ER, Schwartz K, Soto-Torres LE, Govender V, et al. Use of a vaginal ring containing dapivirine for HIV-1 prevention in women. N Engl J Med 2016; 375: 2121-32. [http://dx.doi.org/10.1056/NEJMoa1506110] |
| [28] | Hendrix CW. Exploring concentration response in HIV pre-exposure prophylaxis to optimize clinical care and trial design. Cell 2013; 155: 515-8. [http://dx.doi.org/10.1016/j.cell.2013.09.030] |
| [29] | Veazey RS, Springer MS, Marx PA, Dufour J, Klasse PJ, Moore JP. Protection of macaques from vaginal SHIV challenge by an orally delivered CCR5 inhibitor. Nat Med 2005; 11: 1293-4. [http://dx.doi.org/10.1038/nm1321] |
| [30] | Subbarao S, Otten RA, Ramos A, Kim C, Jackson E, Monsour M. Chemoprophylaxis with tenofovir disoproxil fumarate provided partial protection against infection with simian human immunodeficiency virus in macaques given multiple virus challenges. J Infect Dis 2006; 194: 904-11. [http://dx.doi.org/10.1086/507306] |
| [31] | Gupta K, Klasse PJ. How do viral and host factors modulate the sexual transmission of HIV? Can transmission be blocked? PLoS Med 2006; 3: e79. [http://dx.doi.org/10.1371/journal.pmed.0030079] |
| [32] | Miller CJ, Alexander NJ, Sutjipto S, et al. Genital mucosal transmission of simian immunodeficiency virus: Animal model for heterosexual transmission of human immunodeficiency virus. J Virol 1989; 63: 4277-84. |
| [33] | Pauza CD, Horejsh D, Wallace M. Mucosal transmission of virulent and avirulent lentiviruses in macaques. AIDS Res Hum Retroviruses 1998; 14: S83-7. |
| [34] | Williams R, Bokhari S, Silverstein P, Pinson D, Kumar A, Buch S. Nonhuman primate models of NeuroAIDS. J Neurovirol 2008; 14: 292-300. [http://dx.doi.org/10.1080/13550280802074539] |
| [35] | Wege AK, Melkus MW, Denton PW, Estes JD, Garcia JV. Functional and phenotypic characterization of the humanized BLT mouse model. Curr Top Microbiol Immunol 2008; 324: 149-65. [http://dx.doi.org/10.1007/978-3-540-75647-7_10] |
| [36] | Denton PW, Estes JD, Sun Z, et al. Antiretroviral pre-exposure prophylaxis prevents vaginal transmission of HIV-1 in humanized BLT mice. PLoS Med 2008; 5: e16. [http://dx.doi.org/10.1371/journal.pmed.0050016] |
| [37] | Denton PW, Krisko JF, Powell DA, et al. Systemic administration of antiretrovirals prior to exposure prevents rectal and intravenous HIV-1 transmission in humanized BLT mice. PLoS One 2010; 5: e8829. [http://dx.doi.org/10.1371/journal.pone.0008829] |
| [38] | Denton PW, Othieno F, Martinez-Torres F, et al. One percent tenofovir applied topically to humanized BLT mice and used according to the CAPRISA 004 experimental design demonstrates partial protection from vaginal HIV infection, validating the BLT model for evaluation of new microbicide candidates. J Virol 2011; 85: 7582-93. [http://dx.doi.org/10.1128/JVI.00537-11] |
| [39] | Denton PW, Garcia JV. Mucosal HIV-1 transmission and prevention strategies in BLT humanized mice. Trends Microbiol 2012; 20: 268-74. [http://dx.doi.org/10.1016/j.tim.2012.03.007] |
| [40] | Chateau ML, Denton PW, Swanson MD, McGowan I, Garcia JV. Rectal transmission of transmitted/founder HIV-1 is efficiently prevented by topical 1% tenofovir in BLT humanized mice. PLoS One 2013; 8: e60024. [http://dx.doi.org/10.1371/journal.pone.0060024] |
| [41] | Deruaz M, Luster AD. BLT humanized mice as model to study HIV vaginal transmission. J Infect Dis 2013; 208(Suppl. 2): S131-6. [http://dx.doi.org/10.1093/infdis/jit318] |
| [42] | Wahl A, Victor Garcia J. The use of BLT humanized mice to investigate the immune reconstitution of the gastrointestinal tract. J Immunol Methods 2014; 410: 28-33. [http://dx.doi.org/10.1016/j.jim.2014.06.009] |
| [43] | Karpel ME, Boutwell CL, Allen TM. BLT humanized mice as a small animal model of HIV infection. Curr Opin Virol 2015; 13: 75-80. [http://dx.doi.org/10.1016/j.coviro.2015.05.002] |
| [44] | Melkus MW, Estes JD, Padgett-Thomas A, et al. Humanized mice mount specific adaptive and innate immune response to EBV and TSST-1. Nat Med 2006; 12: 1316-22. [http://dx.doi.org/10.1038/nm1431] |
| [45] | Nischang M, Sutmuller R, Gers-Huber G, et al. Humanized mice recapitulate key features of HIV-1 infection: A novel concept using long-acting anti-retroviral drugs for treating HIV-1. PLoS One 2012; 7: e38853. [http://dx.doi.org/10.1371/journal.pone.0038853] |
| [46] | Denton PW, Othieno F, Martinez-Torres F, et al. One percent tenofovir applied topically to humanized BLT mice and used according to the CAPRISA 004 experimental design demonstrates partial protection from vaginal HIV infection, validating the BLT model for evaluation of new microbicide candidates. J Virol 2011; 85: 7582-93. [http://dx.doi.org/10.1128/JVI.00537-11] |
| [47] | Chateau ML, Denton PW, Swanson MD, McGowan I, Garcia JV. Rectal transmission of transmitted/founder HIV-1 is efficiently prevented by topical 1% tenofovir in BLT humanized mice. PLoS One 2013; 8: e60024. [http://dx.doi.org/10.1371/journal.pone.0060024] |
| [48] | Cheng G, Montero A, Gastaminza P, et al. A virocidal amphipathic {alpha}-helical peptide that inhibits hepatitis C virus infection in vitro. Proc Natl Acad Sci USA 2008; 105: 3088-93. [http://dx.doi.org/10.1073/pnas.0712380105] |
| [49] | Brass V, Bieck E, Montserret R, et al. An amino-terminal amphipathic alpha-helix mediates membrane association of the hepatitis C virus nonstructural protein 5A. J Biol Chem 2002; 277: 8130-9. [http://dx.doi.org/10.1074/jbc.M111289200] |
| [50] | Penin F, Brass V, Appel N, et al. Structure and function of the membrane anchor domain of hepatitis C virus nonstructural protein 5A. J Biol Chem 2004; 279: 40835-43. [http://dx.doi.org/10.1074/jbc.M404761200] |
| [51] | Bobardt MD, Cheng G, de Witte L, et al. Hepatitis C virus NS5A anchor peptide disrupts human immunodeficiency virus. Proc Natl Acad Sci USA 2008; 105: 5525-30. [http://dx.doi.org/10.1073/pnas.0801388105] |
| [52] | Lan P, Tonomura N, Shimizu A, Wang S, Yang YG. Reconstitution of a functional human immune system in immunodeficient mice through combined human fetal thymus/liver and CD34_ cell transplantation. Blood 2006; 108: 487-92. [http://dx.doi.org/10.1182/blood-2005-11-4388] |
| [53] | Sun Z, Denton PW, Estes JD, et al. Intrarectal transmission, systemic infection and CD4. T cell depletion in humanized mice infected with HIV-1. J Exp Med 2007; 204: 705-14. [http://dx.doi.org/10.1084/jem.20062411] |
| [54] | Stoddart CA, Maidji E, Galkina SA, et al. Superior human leukocyte reconstitution and susceptibility to vaginal HIV transmission in humanized NOD-scid IL-2Rγ(-/-) (NSG) BLT mice. Virology 2011; 417: 154-60. [http://dx.doi.org/10.1016/j.virol.2011.05.013] |
| [55] | Chou TC. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol Rev 2006; 58: 621-81. [http://dx.doi.org/10.1124/pr.58.3.10] |
| [56] | Chou TC, Martin N. CompuSyn for drug combinations: PC software and user’s guide: A computer program for quantitation of synergism and antagonism in drug combinations, and the determination of IC50 and ED50 and LD50 values 2005. |
| [57] | de Witte L, Bobardt MD, Chatterji U, et al. HSV neutralization by the microbicidal candidate C5A. PLoS One 2011; 6: e18917. [http://dx.doi.org/10.1371/journal.pone.0018917] |
| [58] | Veazey RS, Chatterji U, Bobardt M, et al. C5A protects macaques from vaginal simian-human immunodeficiency virus challenge. Antimicrob Agents Chemother 2015; 60: 693-8. [http://dx.doi.org/10.1128/AAC.01925-15] |
| [59] | Zeitlin L, Hoen TE, Achilles SL, et al. Tests of Buffergel for contraception and prevention of sexually transmitted diseases in animal models. Sex Transm Dis 2001; 28: 417-23. [http://dx.doi.org/10.1097/00007435-200107000-00010] |
| [60] | Daggett GJ Jr, Zhao C, Connor-Stroud F, et al. Comparison of the vaginal environment in rhesus and cynomolgus macaques pre- and post-lactobacillus colonization. J Med Primatol 2017; 46: 232-8. [http://dx.doi.org/10.1111/jmp.12264] |
| [61] | Shen L, Peterson S, Sedaghat AR, et al. Dose-response curve slope sets class-specific limits on inhibitory potential of anti-HIV drugs. Nat Med 2008; 14: 762-6. [http://dx.doi.org/10.1038/nm1777] |








