- Home
- About Journals
-
Information for Authors/ReviewersEditorial Policies
Publication Fee
Publication Cycle - Process Flowchart
Online Manuscript Submission and Tracking System
Publishing Ethics and Rectitude
Authorship
Author Benefits
Reviewer Guidelines
Guest Editor Guidelines
Peer Review Workflow
Quick Track Option
Copyediting Services
Bentham Open Membership
Bentham Open Advisory Board
Archiving Policies
Fabricating and Stating False Information
Post Publication Discussions and Corrections
Editorial Management
Advertise With Us
Funding Agencies
Rate List
Kudos
General FAQs
Special Fee Waivers and Discounts
- Contact
- Help
- About Us
- Search

The Open Leukemia Journal
(Discontinued)
ISSN: 1876-8164 ― Volume 5, 2013
Stimulation of Toll-Like Receptor-7 Enhances BAFF and APRIL Pathways of Survival in Chronic Lymphocytic Leukemia Cells
Amar Hammadi1, Christian Billard1, Brigitte Bauvois1, Anne-Marie Faussat1, 2, Jean-Pierre Kolb*, 1
Abstract
CLL cells are resistant in vivo to apoptosis, in part, through the autocrine action of the survival factors BAFF and APRIL. We show here, by flow cytometry, that stimulation of Toll-like receptor-7 (TLR-7) with imidazoquinolines induces increases in the expression by CLL cells of BAFF, APRIL and their receptors, results confirmed by Western blot. Moreover, sBAFF and sAPRIL released by leukemia cells are increased in supernatants from Resiquimod-treated cells. These events correlated with an increased resistance against apoptosis, both APRIL and BAFF contributing to this protection. Ligation of TLR-7 activated the canonical and alternative pathways of NFκB activation. An inhibitor of I κBa phosphorylation largely prevented these effects. Addition of Resiquimod to CLL cells also elicited the activation of several members of the AP-1 family, notably phosphorylation of c-Jun, and the protection against apoptosis was mostly reverted with a specific inhibitor of JNK.
TLR-7 signaling therefore stimulates apoptosis resistance through activation of the BAFF/APRIL pathways. TLR-7 may be activated in vivo by PAMPs (pathogen-associated molecular patterns) of microorganisms and/or antigens expressed by cells undergoing apoptosis, as already reported for stimulation of the BCR in CLL cells. The engagement of TLRs could be involved in the aetiology of CLL.
Article Information
Identifiers and Pagination:
Year: 2010Volume: 3
First Page: 24
Last Page: 33
Publisher Id: TOLEUKEMIAJ-3-24
DOI: 10.2174/1876816401003010024
Article History:
Received Date: 28/05/2009Revision Received Date: 19/10/2009
Acceptance Date: 20/10/2009
Electronic publication date: 29/1/2010
Collection year: 2010
open-access license: This is an open access article licensed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/bync/3.0/) which permits unrestricted, non-commercial use, distribution and reproduction in any medium, provided the work is properly cited.
* Address correspondence to this author at the UMRS 872, Team 18, Centre de Recherche des Cordeliers, 15 rue de l’Ecole de Medecine, 75270 Paris cedex 06, France; Tel/Fax: +33 (0)1 43 25 63 44; E-mail: jean-pierre.kolb@crc.jussieu.fr
| Open Peer Review Details | |||
|---|---|---|---|
| Manuscript submitted on 28-05-2009 |
Original Manuscript | Stimulation of Toll-Like Receptor-7 Enhances BAFF and APRIL Pathways of Survival in Chronic Lymphocytic Leukemia Cells | |
INTRODUCTION
Chronic lymphocytic leukemia (CLL) is the most frequent leukemia in Western countries and still remains an incurable disease, despite recent progress [1Caligaris-Cappio F, Ghia P. Novel insights in chronic lymphocytic leukemia are we getting closer to understanding the pathogenesis of the disease J Clin Oncol 2008; 26: 4497-503.]. It affects mostly people over 60 years and consists in the accumulation in the blood and lymphoid organs of a mono- or oligoclonal population of CD5+ B lymphocytes. About 80% of the patients exhibit various cytogenetic alterations, yet CLL cells do not express a typical translocation. A small pool of highly proliferating cells has been identified in the bone marrow and lymph nodes that feed the blood compartment [2Messmer BT, Messmer D, Allen SL. In vivo measurements document the dynamic cellular kinetics of chronic lymphocytic leukemia B cells J Clin Invest 2005; 115: 755-64.]. The latter pool consists in anergic and non-dividing small B-lymphocytes, 95-98% of them being arrested in the G0/G1 phase of the cell cycle. Although these cells are highly resistant to apoptosis in vivo, they become sensitive when cultured ex vivo and die rather rapidly, unless they are incubated in the presence of stromal cells that can rescue them from programmed cell death. This suggests that the microenvironment protect them from apoptosis in vivo and therefore CLL is a disease of both proliferation and accumulation. A hallmark of CLL cells resides in their dramatic resistance to both spontaneous and drug-induced apoptosis. Several mechanisms that prevent leukemia cells or rescue them from entering the process of programmed cell death have been identified, yet the early signal conferring apoptosis resistance to the tumor cells are still poorly understood.
However, there is now a growing consensus to incriminate an interaction between CLL precursor cells with self-antigens and/or microbial antigens in the aetiology of this leukemia. Indeed, cells from a significant fraction of CLL patients display strikingly similar BCR, indicating that these BCRs, resembling those of mAb’s reactive with carbohydrates of bacterial capsules or viral coats and with certain auto antigens, could bind the same antigenic epitopes. These findings suggested that the B-lymphocytes that gave rise to these leukemia cells were selected for this unique BCR structure [3Ghiotto F, Fais F, Valetto A. Remarkably similar antigen receptors among a subset of patients with chronic lymphocytic leukemia J Clin Invest 2004; 113: 1008-6.]. The relatively high frequency in CLL patients (about 20%) of these so-called stereotyped receptors imply that either a significant fraction of CLL cells was selected by a restricted number of antigenic epitopes during their development and/or that they derive from a distinct B cell subpopulation with limited Ig V region diversity [4Messmer BT, Albesiano E, Efremov DG. Multiple distinct sets of stereotyped antigen receptors indicate a role for antigen in promoting chronic lymphocytic leukemia J Exp Med 2004; 200: 519-25.]. CLL cells very frequently express BCR with a monoclonal immunoglobulin displaying characteristic features of antibodies against self-antigens or against microbial antigens [5Chiorazzi N, Hatzi K, Albesiano E. B-cell chronic lymphocytic leukemia a clonal disease of B lymphocytes with receptors that vary in specificity for (auto) antigens Ann NY Acad Sci 2005; 1062: 1-12.].
Recently, several antigens binding to monoclonal Ig from various CLL patients and cell lines have been identified and included molecular motifs exposed on apoptotic cells/blebs and bacteria [6LanemoMyhrinder A, Hellqvist E, Sidorova E. A new perspective molecular motifs on oxidized LDL apoptotic cells and bacteria are targets for chronic lymphocytic leukemia antibodies Blood 2008; 111: 3838-48.]. Their results showed limited target structure recognition and indicated that CLL B cells derive from a cell compartment that produces "natural antibodies," aimed at the elimination and scavenging of apoptotic cells and pathogenic bacteria. Clonal expansion in CLL may thus be stimulated by auto antigens occurring during apoptosis, some of the latter epitopes being similar to those on bacteria and microbes [7Catera R, Silverman GJ, Hatzi K. Chronic lymphocytic leukemia cells recognize conserved epitopes associated with apoptosis and oxidation Mol Med 2008; 14: 665-74.]. These data suggest that CLL may derive from normal B cells whose function is to remove cellular debris, and also to provide a first line of defence against pathogens. Moreover, several lines of evidence suggest that the encounter of the leukemia cells (or their precursors) with such antigens might be involved in the oncogenic process and is a driving force for the proliferation and differentiation of the tumor cells [4Messmer BT, Albesiano E, Efremov DG. Multiple distinct sets of stereotyped antigen receptors indicate a role for antigen in promoting chronic lymphocytic leukemia J Exp Med 2004; 200: 519-25., 8Murray F, Darzentas N, Hadzidimitriou A. Stereotyped patterns of somatic hypermutation in subsets of patients with chronic lymphocytic leukemia implications for the role of antigen selection in leukemogenesis Blood 2008; 111: 1524-33.].
The nature of the antigen(s) binding the BCR of CLL cells therefore strongly suggests that the structures bearing these antigenic motifs may be also recognized by the receptors in charge of innate immunity, i.e. the Toll-like receptors or TLR [9Akira S, Takeda K. Toll-like receptor signalling Nat Rev Immunol 2004; 4: 499-511.]. Several functional TLR are present at the membrane or in the cytosol of CLL cells [10Grandjenette C, Kennel A, Faure GC. Expression of functional toll-like receptors by B-chronic lymphocytic leukemia cells Haematologica 2007; 92: 1279-81., 11Muzio M, Scielzo C, Bertilaccio MT. Expression and function of toll like receptors in chronic lymphocytic leukemia cells Br J Haematol 2009; 144: 507-16.]. TLR recognize PAMPs (pathogen-associated molecular patterns) and their engagement lead to NFκB activation [9Akira S, Takeda K. Toll-like receptor signalling Nat Rev Immunol 2004; 4: 499-511., 12Barton GM, Medzhitov R. Toll-like receptor signaling pathways Science 2003; 300: 1524-25., 13Peng SL. Signaling in B cells via toll-like receptors Curr Opin Immunol 2005; 17: 230-6.]. We thus made the hypothesis that the stimulation of TLR by PAMPs/autoantigens displayed by autologous dying cells and/or foreign micro-organisms might contribute, in addition to BCR engagement, to the survival of the leukemic clone. Indeed, we showed recently that addition of Resiquimod, a specific TLR-7 ligand, to CLL cells resulted in a NFκB dependent up-regulation of the inducible nitric oxide synthase (iNOS) and of NO production [14Hammadi A, Billard C, Faussat AM. Stimulation of iNOS expression and apoptosis resistance in B-cell chronic lymphocytic leukemia (B-CLL) cells through engagement of Toll-like receptor 7 (TLR-7) and NF-?B activation Nitric Oxide 2008; 19: 138-45.]. Several years ago, we demonstrated that NO released by an endogenous iNOS contributed to the resistance of the leukemia cells to apoptosis [15Zhao H, Dugas N, Mathiot C. B-cell chronic lymphocytic leukemia cells express a functional inducible nitric oxide synthase displaying anti-apoptotic activity Blood 1998; 92: 1031-43.], mainly through the inhibitory effect of NO on the active site of caspases [16Kolb JP. Mechanisms involved in the pro- and anti-apoptotic role of NO in human leukemia Leukemia 2000; 14: 1685-94.].
We reported previously that BAFF and APRIL contribute, through paracrine and autocrine pathways, to the enhanced resistance of CLL cells to apoptosis [17Kern C, Cornuel JF, Billard C. Involvement of BAFF and APRIL in the resistance to apoptosis of B-CLL through an autocrine pathway Blood 2004; 103: 679-88.]. In the present work, we made the hypothesis that the stimulation of TLR-7 by specific agonists could stimulate the expression of BAFF and APRIL, therefore leading to an enhanced survival of CLL cells resulting from an elevated resistance to apoptosis. Indeed, our experiments indicate that ligation of TLR-7 results in an enhanced expression of BAFF and APRIL and of the specific BAFF receptor, BAFF-R, that parallels an increased survival of the leukemia cells. These data reinforce the importance of the stimulation of TLR by auto antigens and/or micro-organisms-derived antigens in the induction of apoptosis resistance in CLL cells
MATERIALS AND METHODOLOGY
Patients, Cells and Cells Cultures
CLL patients’ blood samples were obtained from the Hematology Department of Hôtel-Dieu hospital (Paris, France) after informed consent of the patients, in agreement with the revised Helsinki protocol rules. Diagnosis was established according to standard clinical criteria and to the international workshop on CLL (IWCLL), including lymphocyte morphology and co-expression of CD5, CD20 and CD23 antigens. A total of 19 patients (12 men and 7 women) with a mean age of 67 ± 9 years (range 50-85 years) were tested. The time since diagnosis varied between 0 (newly diagnosed patients) and 9 years. All patients were Binet stage A and were previously untreated. The leukemia B cells were isolated as previously described [15Zhao H, Dugas N, Mathiot C. B-cell chronic lymphocytic leukemia cells express a functional inducible nitric oxide synthase displaying anti-apoptotic activity Blood 1998; 92: 1031-43.] with purity greater than 96%.
All cell cultures were carried out in RPMI 1640 medium supplemented with 2 mM glutamine, 1 mM sodium pyruvate, 100 IU/ml penicillin, 100 µg/ml streptomycin and 10% FCS (PAA Laboratories, Les Mureaux, France) at 37°C in an humidified atmosphere containing 5 % CO2. Freshly isolated CLL cells were seeded at 2x106/ml. The MEC-1 cell line, established from the peripheral blood of a 61-year-old man with CLL in prolymphocytic transformation [18Stacchini A, Aragno M, Vallario A. MEC1 and MEC2 two new cell lines derived from B-chronic lymphocytic leukemia in prolymphocytoid transformation Leuk Res 1999; 23: 127-36.], was purchased from DSMZ (Braunschweig, Germany). These cells display a similar pattern of TLR expression as leukemia cells freshly isolated from CLL patients [11Muzio M, Scielzo C, Bertilaccio MT. Expression and function of toll like receptors in chronic lymphocytic leukemia cells Br J Haematol 2009; 144: 507-16.].
Reagents
The TLR-7 agonists Imiquimod/R-837 1-(2-Methyl-propyl)-1H-imidazo4,5-cquinolin-4-amine [19Hemmi H, Kaisho T, Takeuchi O. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway Nat Immunol 2002; 2: 196-200.] and Resi-quimod/R-848 4-Amino-2-(ethoxymethyl)-a,a-dimethyl-1H- imidazo(4,5-c)quinoline-1-ethanol [20Jurk M, Heil F, Vollmer J. Human TLR7 or TLR8 independently confer responsiveness to the antiviral compound R-848 Nat Immunol 2002; 3: 499-501.] were obtained from Axxora (San Diego, CA, USA). These reagents were dis-solved in DMSO as 1 mM stock solutions and kept at -20°C until use. Wedelolactone (7-Methoxy-5,11,12-trihydroxy-coumestan), a furanocoumarin isolated from Eclipta prostrata and a powerful specific inhibitor of the IκB kinase (IKK) complex [21Kobori MZ, Yang Z, Gong D. Wedelolactone suppresses LPS-induced caspase-11 expression by directly inhibiting the IKK complex Cell Death Differ 2004; 11: 123-30.] was purchased from Biomol (Enzo Life Sciences, Plymouth Meeting, PA, USA). Human BCMA-Fc was a kind gift from Dr A. Tsapis (Créteil). Recombinant human BAFF-R-Fc chimera was purchased from R &D (Abingdon, UK). The potent cell-permeable selective and reversible inhibitor of c-Jun N-terminal kinase, JNK Inhibitor II or Anthra(1,9-cd)pyrazol-6(2H)-one, that blocks the phosphorylation of c-jun [22Shin M, Yan C, Boyd D. An inhibitor of c-jun aminoterminal kinase (SP600125) represses c-Jun activation DNA-binding and PMA-inducible 92-kDa type IV collagenase expression Biochim Biophys Acta 2002; 1589: 311-6.] was purchased from Calbiochem (Merck Chemicals, Nottingham, UK).
Flow Cytometry Analysis
The expression of BAFF, APRIL and their receptors at the membrane of CLL cells was analyzed by indirect immunofluorescence and flow cytometry. The following goat polyclonal IgG were used: TALL-1(BAFF) (Y-15 sc-5743) and TALL-1 (C-16 sc-5744) ; APRIL (R-15 sc-5739) ; BCMA (N-16 sc-11743) ; TACI (C-20 sc7332) and TACI (N-19 sc-7333), all from Santa Cruz Biotechnology (Tebu-Bio, Le Perray en Yvelines, France) and anti-hBAFF-R from R&D (AF 1162). Purified rabbit IgG (Sigma Aldrich, Saint Quentin Fallavier, France) was used as isotype control and FITC-conjugated affinipure F(ab’)2 fragment donkey anti-goat IgG(H+L) (Jackson Immunoresearch, Interchim, Montlucon, France) was used as second antibody. In addition, membrane BAFF and BAFF-R were also detected by direct fluorescence with fluorescein conjugated mouse IgG1 mAb anti-hBAFF (R&D IC1241F) and anti-hBAFF-R (Abcam mAb38977) respectively, using FITC mouse IgG1 as isotype control (Becton Dickinson BD555748, BD, Le Pont de Claix, France). For the detection of TLR-7, cells were permeabilized with the CytoFix/CytoPerm kit (Becton-Dickinson) and incubated with a mouse monoclonal anti TLR-7 antibody (IgG1κ; clone 4F4) (Axxara) or mouse IgG1 (Jackson Immunoresearch) as control; binding was revealed with a fluorescein-conjugated, affinity-purified goat F(ab’)2 fragment anti-mouse IgG (H+L) (Rockland, Gilbertsville, PA). Cells were analysed by flow cytometry (EPICS Altra or Coulter Epics XL, Beckman Coulter France, Roissy, France) using the CellQuest program. The estimation of the D-values (roughly equivalent to the percentage of positive cells) resulting from the subtraction of the histogramme of the isotypic control from the histogramme of the test antibody, was performed according to the technique of Kolmogorov-Smirnov. In some instances, the ∆ MFI (differences between the mean fluorescence intensity of test and control antibody) were also presented.
NFκB Activation
Activation of the different NFκB members was measured with the TransAM NFκB family ELISA kit (Active Motif, Carlsbad, CA), according to the specifications of the manufacturer. Briefly this test is based on the specific binding of activated NFκB components contained in nuclear extracts to a κB consensus site (5´-GGGACTTTCC-3´) that has been immobilized in the wells of a microtitration plate. After treatment with Resiquimod or Imiquimod for 6-8 h, B-CLL cells were lysed using the “nuclear extract kit” from Active Motif according to the manufacturer and protein content was quantified using the Bradford assay. The binding was monitored with antibodies recognizing epitopes on p50, p52, p65, c-Rel or RelB subunits that are accessible only when NFκB is activated and bound to its target DNA, followed by an HRP-conjugated secondary antibody and colorimetric readout at 405 nm with a WallacVictor 2 (Perkin Elmer, Courtaboeuf, France) microplate spectrophotometer.
AP-1 Activation
The quantification in nuclear lysates of the various members of the AP-1 family of transcription factors was performed in a similar way as for NFκB with the TransAM AP-1 Family Kit (Active Motif), according to the specifications of the manufacturer.
DNA Fragmentation Assay
Apoptosis was also quantified by DNA fragmentation as evaluated by detection of cytoplasmic histone-associated DNA fragments (mono- and oligonucleosomes) in cell lysates from aliquots of 20,000 cells using an ELISA with anti-histone and anti-DNA fragment mAbs (Cell Death Detection ELISAPLUS, Roche Diagnostics, Indianapolis, IN, USA) as previously described [14Hammadi A, Billard C, Faussat AM. Stimulation of iNOS expression and apoptosis resistance in B-cell chronic lymphocytic leukemia (B-CLL) cells through engagement of Toll-like receptor 7 (TLR-7) and NF-?B activation Nitric Oxide 2008; 19: 138-45.].
Caspase 8 Activity
It was measured as described previously [14Hammadi A, Billard C, Faussat AM. Stimulation of iNOS expression and apoptosis resistance in B-cell chronic lymphocytic leukemia (B-CLL) cells through engagement of Toll-like receptor 7 (TLR-7) and NF-?B activation Nitric Oxide 2008; 19: 138-45.]. Briefly, aliquots of cell lysates (20 µg) were incubated with the fluorogenic caspase-8 substrate IETD-aminocoumarin, IETD-AMC (caspase-8 cellular assay, Biomol). The release of AMC was recorded every 10 min during 80 min in a Victor-2 microplate reader thermostated at 37°C (excitation wavelength: 380 nm; emission wavelength: 460 nm). The specificity of the reaction was assessed by the addition of unlabeled Ac-IETD-CHO caspase-8 inhibitor (Biomol).
Western Blot Analysis
The expression of BAFF and BAFF-R was studied by Western blotting, as detailed previously [23Billard C, Kern C, Tang R, Ajchenbaum-Cymbalista F, Kolb JP. Flavopiridol downregulates the expression of both the inducible NO synthase and p27kip1 in malignant cells from B-cell chronic lymphocytic leukemia Leukemia 2003; 17: 2435-43.]. Briefly, equal amounts of the cell lysates (generally 30-50 μg of proteins in SDS-reducing buffer) were electrophoresed by SDS-PAGE (8%). The goat polyclonal IgG against BAFF/TALL-1 (C-16 sc-5744, Santa Cruz Biotechnology, CA, USA) and anti-hBAFF-R (AF 1162, R&D) were used for the detection and the immunoblotted proteins were revealed with a polyclonal HRP-coated rabbit anti-goat antibody (DakoCymation, Glostrup, Denmark) and a system of chemiluminescence (Western lightning chemiluminescence reagent plus; Perkin Elmer, Boston, MA, USA). Finally, the membranes were also hybridized with a mouse anti-b actin mAb (clone C4; ICN, Costa Mesa, CA, USA) for protein content monitoring and standardization. Films were analyzed with a digital imager (Vilber Lourmat, Marne-la-Vallée, France) system and the NIH Image 1.44b11 software was used for the quantification of the intensity of the bands.
Quantification of Soluble BAFF and APRIL (sBAFF and sAPRIL) in Cell supernatants
CLL cells were adjusted at 4 x 106/ml in RPMI-1640 medium and allowed to incubate for 48 h in the presence or not of varying concentrations of Resiquimod. After centrifugation of the cells, the supernatants were collected and the levels of sBAFF estimated by a specific ELISA (Quantikine human BAFF/BlyS, R&D, Abingdon, UK) as previously described [24Haiat S, Billard C, Quiney C. Role of BAFF and APRIL in human B-cell chronic lymphocytic leukemia, B-CLL Immunology 2006; 118: 281-92.]. In parallel, sAPRIL was measured with the human APRIL ELISA kit (AbCys, Paris, France) according to the specifications of the manufacturer. Recombinant soluble BAFF (amino acids 134-285, 17 kDa) was from BioVision Research Products (Mountain View, CA).
Statistical Analysis
Experiments were performed with duplicate or triplicate cell cultures. Statview software was used for statistical analysis. Test and control groups were compared using the unpaired two tail Student’s t test.
RESULTS
Enhanced Survival of CLL Cells Following the Ligation of TLR-7
We showed previously that incubation of CLL cells with two specific ligands of TLR-7, Resiquimod and Imiquimod, improved their viability after three days of culture ex vivo [14Hammadi A, Billard C, Faussat AM. Stimulation of iNOS expression and apoptosis resistance in B-cell chronic lymphocytic leukemia (B-CLL) cells through engagement of Toll-like receptor 7 (TLR-7) and NF-?B activation Nitric Oxide 2008; 19: 138-45.]. This pro-survival effect of the engagement of TLR-7 results from a decreased spontaneous apoptosis, as attested by a marked reduction in cytoplasmic nucleosomes, a surrogate for internucleosomal DNA cleavage (Fig. 1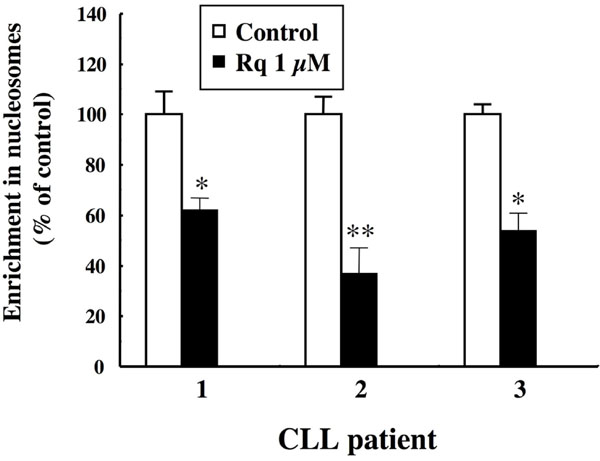 ). Treatment of the CLL cells of three patients for 48 h with 1 μM Resiquimod was found to result in a significant decrease (range 40-60%) in the enrichment of cytoplasmic nucleosomes. Similar results were obtained with Imiquimod (not shown). This was accompanied by a marked reduction in the activity of caspase 8, as shown in Fig. (2
). Treatment of the CLL cells of three patients for 48 h with 1 μM Resiquimod was found to result in a significant decrease (range 40-60%) in the enrichment of cytoplasmic nucleosomes. Similar results were obtained with Imiquimod (not shown). This was accompanied by a marked reduction in the activity of caspase 8, as shown in Fig. (2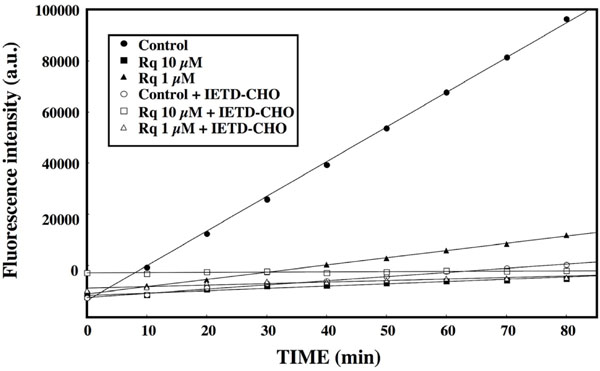 ), where incubation of CLL cells with Resiquimod resulted in a dose-dependent decrease of the cleavage of the caspase 8 specific substrate IETD-AMC. Altogether, these data confirm that the engagement of TLR-7 leads to an early enhanced viability and resistance of the leukemia cells to spontaneous apoptosis.
), where incubation of CLL cells with Resiquimod resulted in a dose-dependent decrease of the cleavage of the caspase 8 specific substrate IETD-AMC. Altogether, these data confirm that the engagement of TLR-7 leads to an early enhanced viability and resistance of the leukemia cells to spontaneous apoptosis.
Ligation of TLR-7 Stimulates the Expression of BAFF, APRIL by CLL Cells
We hypothesized that the enhanced viability of the CLL cells resulting from TLR-7 engagement could be due, in part, to the induction of a response of these cells to pro-survival factors, such as BAFF and APRIL. We thus tested the expression of BAFF and APRIL at the surface of the leukemia cells. As shown in Fig. (3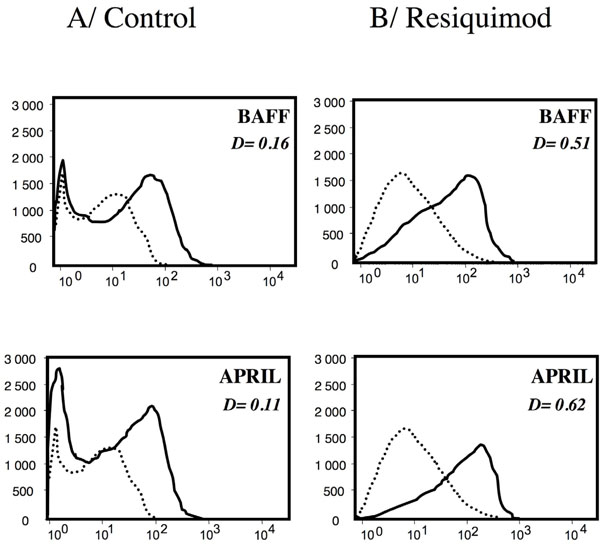 ) for one representative CLL patient, BAFF and its the closely related APRIL were detected at low but significant levels at the membrane of these cells, in agreement with our previous report [17Kern C, Cornuel JF, Billard C. Involvement of BAFF and APRIL in the resistance to apoptosis of B-CLL through an autocrine pathway Blood 2004; 103: 679-88.]. Incubation of the cells with Resiquimod 1μM was found to result in an enhanced expression of BAFF and APRIL as shown in Fig. (3
) for one representative CLL patient, BAFF and its the closely related APRIL were detected at low but significant levels at the membrane of these cells, in agreement with our previous report [17Kern C, Cornuel JF, Billard C. Involvement of BAFF and APRIL in the resistance to apoptosis of B-CLL through an autocrine pathway Blood 2004; 103: 679-88.]. Incubation of the cells with Resiquimod 1μM was found to result in an enhanced expression of BAFF and APRIL as shown in Fig. (3 ). These increases were highly significant as estimated by Kolmogorov-Smirnov. Comparable results were observed with the MEC-1 cell line derived from a B-CLL patient, as shown in Fig. (4
). These increases were highly significant as estimated by Kolmogorov-Smirnov. Comparable results were observed with the MEC-1 cell line derived from a B-CLL patient, as shown in Fig. (4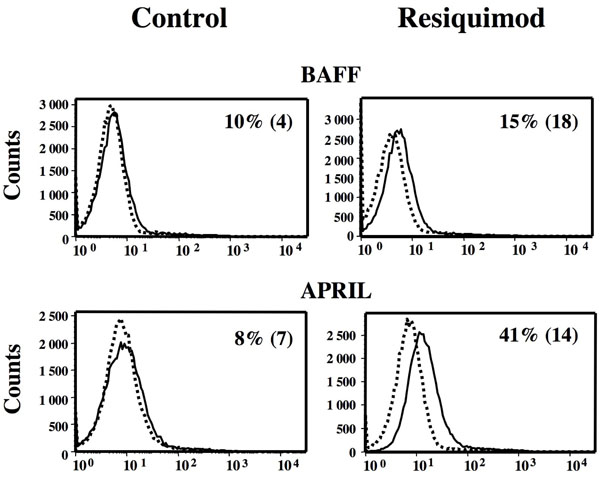 ). Although the spontaneous expression of BAFF and APRIL by MEC-1 cells was lower than that displayed by primary CLL cells, it was still significantly increased in the presence of Resiquimod.
). Although the spontaneous expression of BAFF and APRIL by MEC-1 cells was lower than that displayed by primary CLL cells, it was still significantly increased in the presence of Resiquimod.
Ligation of TLR-7 Stimulates BAFF-R Expression and is Dependent on NFκB Activation
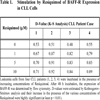
The specific receptor for BAFF, BAFF-R, was also found to increase following stimulation of TLR-7 with Resiquimod, as detected by FACS analysis. In comparison with BAFF and APRIL, the expression of BAFF-R, already high in unstimulated leukemia cells (as previously described [24Haiat S, Billard C, Quiney C. Role of BAFF and APRIL in human B-cell chronic lymphocytic leukemia, B-CLL Immunology 2006; 118: 281-92.]), is further increased significantly after incubation with Resiquimod (Fig. 5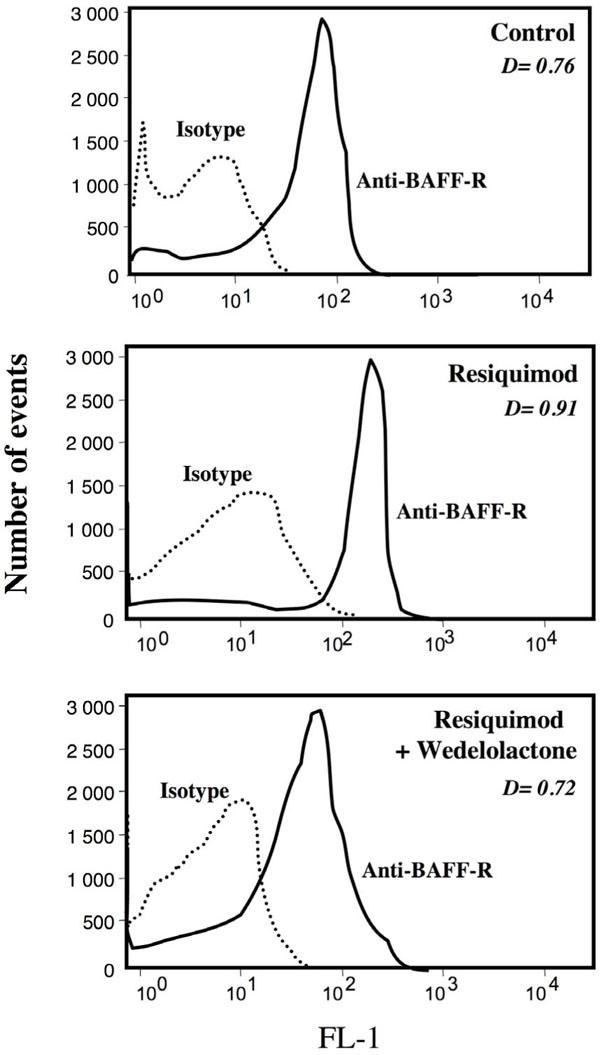 ). Resiquimod-driven increase in BAFF-R expression was almost maximum at 1μM, a plateau of stimulation being observed at higher concentrations (Table 1). Similar data were obtained with Imiquimod (not shown). The augmentation in membrane BAFF-R was largely reverted in the presence of wedelolactone, an inhibitor of the IκB kinase (IKK) complex and of NFκB activation (Fig. 5
). Resiquimod-driven increase in BAFF-R expression was almost maximum at 1μM, a plateau of stimulation being observed at higher concentrations (Table 1). Similar data were obtained with Imiquimod (not shown). The augmentation in membrane BAFF-R was largely reverted in the presence of wedelolactone, an inhibitor of the IκB kinase (IKK) complex and of NFκB activation (Fig. 5 ). The latter treatment was found to result in a loss of the protective effect against apoptosis afforded by Resiquimod alone (not shown).
). The latter treatment was found to result in a loss of the protective effect against apoptosis afforded by Resiquimod alone (not shown).
Western Blot Study of BAFF and BAFF-R in CLL Cells Following Engagement of TLR-7
The elevated expression of BAFF in CLL cells following Resiquimod treatment was further confirmed by Western blotting. Dose-dependent effects of Resiquimod and of another TLR-7 ligand, Isiquimod, on BAFF expression are depicted in Fig. (6A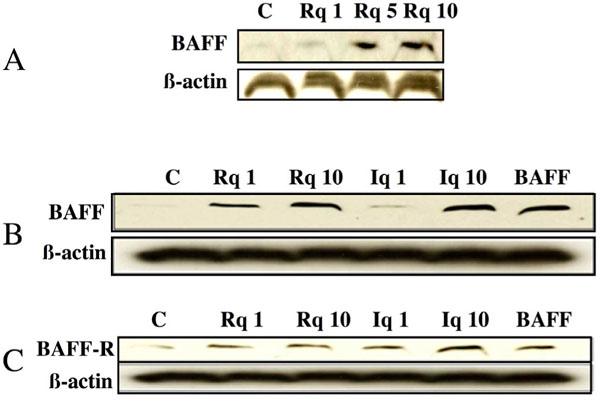 , B
, B ). In parallel, the presence of BAFF-R on CLL cells is increased following incubation with both TLR-7 ligands (Fig. 6C
). In parallel, the presence of BAFF-R on CLL cells is increased following incubation with both TLR-7 ligands (Fig. 6C ). Interestingly, BAFF and BAFF-R expressions also appear to be up regulated by BAFF itself (Fig. 6B
). Interestingly, BAFF and BAFF-R expressions also appear to be up regulated by BAFF itself (Fig. 6B , C
, C ).
).
TLR-7 Stimulation Favors the Release of sBAFF and sAPRIL by CLL Cells
The enhanced expression of BAFF and APRIL was associated with an increased release of their soluble form, sBAFF and sAPRIL, in the supernatants of Resiquimod-treated cells (Fig. 7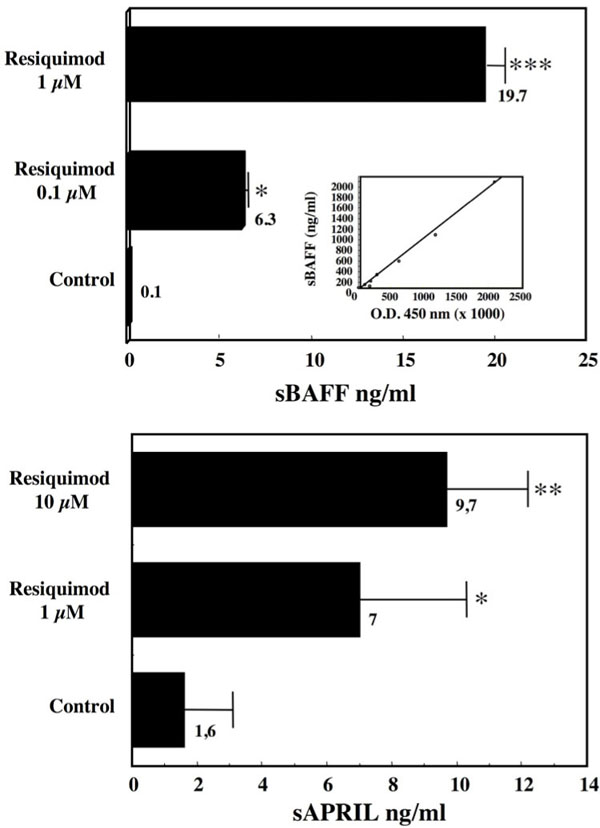 ). In contrast, the expression of the two other BAFF receptors, TACI and BCMA, that are also detected albeit to lower levels than BAFF-R on CLL cells [24Haiat S, Billard C, Quiney C. Role of BAFF and APRIL in human B-cell chronic lymphocytic leukemia, B-CLL Immunology 2006; 118: 281-92.], was roughly unaffected by Resiquimod (not shown).
). In contrast, the expression of the two other BAFF receptors, TACI and BCMA, that are also detected albeit to lower levels than BAFF-R on CLL cells [24Haiat S, Billard C, Quiney C. Role of BAFF and APRIL in human B-cell chronic lymphocytic leukemia, B-CLL Immunology 2006; 118: 281-92.], was roughly unaffected by Resiquimod (not shown).
TLR-7-Driven BAFF and APRIL Increases Contribute Both to the Protective Effect on Apoptosis
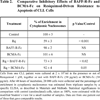
The contribution of the augmented expression and release of BAFF and APRIL to the protection against apoptosis afforded by TLR-7 stimulation was investigated by using neutralizing soluble forms of their receptors. As seen in Table 2, the decrease in spontaneous apoptosis induced by Resiquimod was largely, but not totally, abrogated in the presence of an excess of BCMA-Fc that neutralizes both BAFF and APRIL. In contrast, the protection was only partially alleviated in the presence of BAFF-R-Fc that neutralizes BAFF but not APRIL. These results indicate that the protective effects of the TLR-7 ligand are dependent on the increases in both APRIL and BAFF and some additional factors might also be involved.
TLR-7 Stimulates NFκB Activation in CLL Cells
NFκB activation has been described as a major component of TLR signalling. Accordingly, we observed that the ligation of TLR-7 with Resiquimod resulted in a stimulation of several members of the NF-κB family of transcription factors in CLL cells, as shown in Fig. (8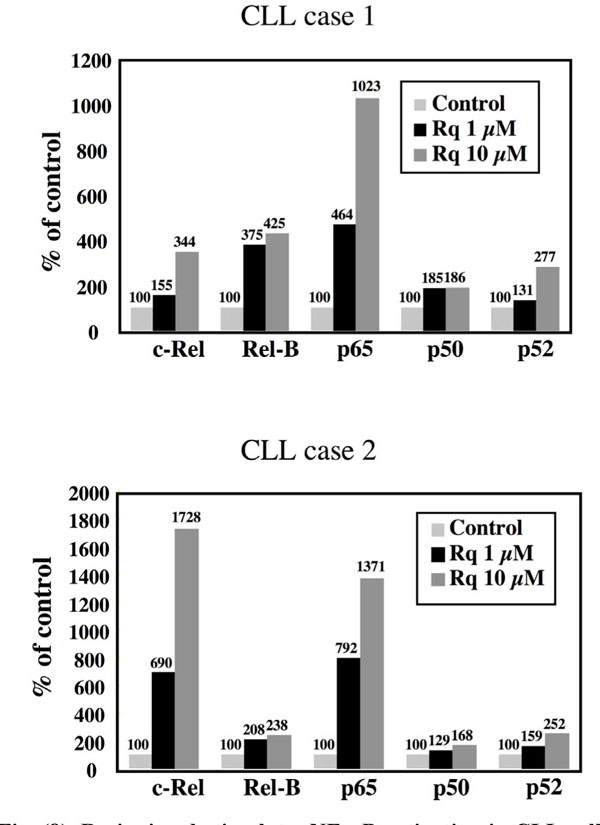 ) for two patients. Both the classical and non-canonical pathways of activation appear to be triggered, inasmuch as significant increases in the nuclear expression of p65/RelA and of p52 could be detected. We previously reported that Resiquimod elicited a dose-dependent phosphorylation of the inhibitor IκBa, leading to its subsequent ubiquitination and degradat-ion by the proteasome [14Hammadi A, Billard C, Faussat AM. Stimulation of iNOS expression and apoptosis resistance in B-cell chronic lymphocytic leukemia (B-CLL) cells through engagement of Toll-like receptor 7 (TLR-7) and NF-?B activation Nitric Oxide 2008; 19: 138-45.].
) for two patients. Both the classical and non-canonical pathways of activation appear to be triggered, inasmuch as significant increases in the nuclear expression of p65/RelA and of p52 could be detected. We previously reported that Resiquimod elicited a dose-dependent phosphorylation of the inhibitor IκBa, leading to its subsequent ubiquitination and degradat-ion by the proteasome [14Hammadi A, Billard C, Faussat AM. Stimulation of iNOS expression and apoptosis resistance in B-cell chronic lymphocytic leukemia (B-CLL) cells through engagement of Toll-like receptor 7 (TLR-7) and NF-?B activation Nitric Oxide 2008; 19: 138-45.].
Activation of AP-1 Family Members by the Engagement of TLR-7
The addition of Resiquimod to CLL cells also stimulates the activation of several members of the AP-1 family of transcription factors. As seen in Fig. (9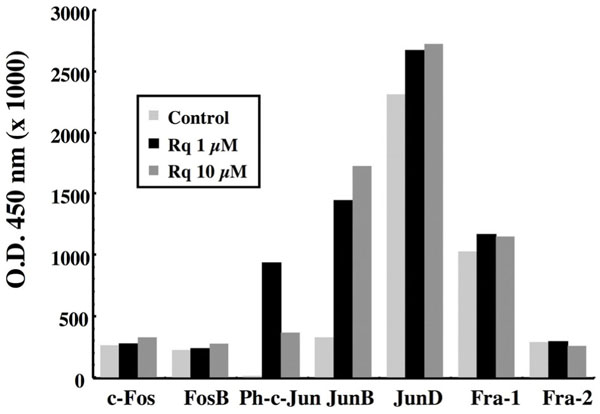 ), the TLR-7 ligand induced a marked increase in the nuclear expression of Jun-B and in the phosphorylated form of c-Jun. A small augmentation in the nuclear expression of Jun-D already spontaneously elevated was also detected, whereas the other members, such as c-Fos, FosB, Fra-1 and Fra-2 were roughly unaffected. Addition of JNK II inhibitor, an inhibitor of c-Jun phosphorylation, largely reversed the protection toward apoptosis afforded by Resiquimod, as shown by return to basal levels of the enrichment in cytoplasmic nucleosomes (Fig. 10
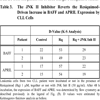
), the TLR-7 ligand induced a marked increase in the nuclear expression of Jun-B and in the phosphorylated form of c-Jun. A small augmentation in the nuclear expression of Jun-D already spontaneously elevated was also detected, whereas the other members, such as c-Fos, FosB, Fra-1 and Fra-2 were roughly unaffected. Addition of JNK II inhibitor, an inhibitor of c-Jun phosphorylation, largely reversed the protection toward apoptosis afforded by Resiquimod, as shown by return to basal levels of the enrichment in cytoplasmic nucleosomes (Fig. 10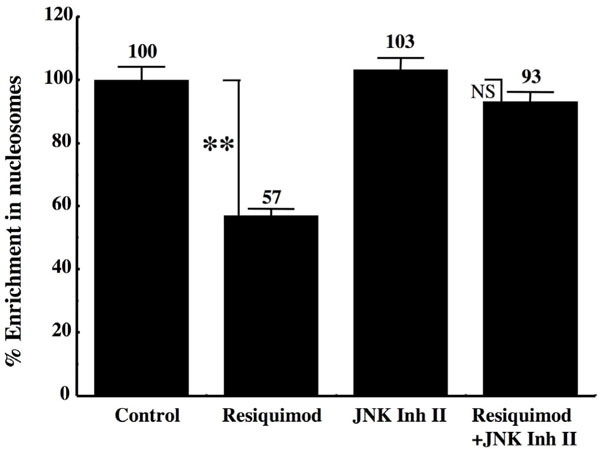 ). The Resiquimod-driven increase in the expression of BAFF and APRIL was also largely counteracted in the presence of this inhibitor (Table 3), emphasizing the link between the activation of AP-1 and the expression of these survival factors.
). The Resiquimod-driven increase in the expression of BAFF and APRIL was also largely counteracted in the presence of this inhibitor (Table 3), emphasizing the link between the activation of AP-1 and the expression of these survival factors.
DISCUSSION
The engagement of TLR-7 with a specific ligand such as Resiquimod was found to enhance the viability of the leukemia cells and to protect them from spontaneous apoptosis. This was accompanied by a decreased activity of caspases 8 and, as already reported [14Hammadi A, Billard C, Faussat AM. Stimulation of iNOS expression and apoptosis resistance in B-cell chronic lymphocytic leukemia (B-CLL) cells through engagement of Toll-like receptor 7 (TLR-7) and NF-?B activation Nitric Oxide 2008; 19: 138-45.], of the effector caspase 3. In this previous work, we showed that the protection afforded by TLR-7 engagement was due, in part, to the up-regulation of the iNOS in these cells and was reduced in the presence of specific iNOS inhibitors [14Hammadi A, Billard C, Faussat AM. Stimulation of iNOS expression and apoptosis resistance in B-cell chronic lymphocytic leukemia (B-CLL) cells through engagement of Toll-like receptor 7 (TLR-7) and NF-?B activation Nitric Oxide 2008; 19: 138-45.]. Here we show, in addition, that stimulation of TLR-7 leads to an up-regulation of two major CLL survival factors, BAFF and APRIL. Both the total amount and the membrane expression of these factors were increased. Moreover, the expression of the specific receptor for BAFF, BAFF-R was also increased by TLR-7 stimulation. This suggested that the enhanced resistance to apoptosis conferred to CLL cells by TLR-7 stimulation could be due to an increased efficiency of the BAFF and APRIL autocrine survival pathways. This hypothesis was supported by the observation that the neutralizing BCMA-Fc and BAFF-R-Fc significantly reversed the protective effect resulting from TLR-7 ligation.
TLR-7 has been reported to signal mainly through the activation of the MyD88/NF-κB pathway [19Hemmi H, Kaisho T, Takeuchi O. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway Nat Immunol 2002; 2: 196-200., 25Huyton T, Rossjohn J, Wilce M. Toll-like receptors: structural pieces of a curve-shaped puzzle Immunol Cell Biol 2007; 85: 406-10., 26Banerjee A, Gerondakis S. Coordinating TLR-activated signaling pathways in cells of the immune system Immunol Cell Biol 2007; 85: 420-.]. In agreement, we found that TLR-7 engagement resulted in the activation of several NF-κB components, notably p65/RelA and p52, suggesting that both the canonical and alternative pathways were stimulated. Inasmuch as the BAFF gene exhibits several κB responsive elements in its promoter region [27Moon EY, Park H. B cell activating factor (BAFF) gene promoter activity depends upon co-activator p300 Immunobiology 2007; 212: 637-45.] and is transcriptionally regulated by NF-κB dependent mechanism [28Tai YT, Li XF, Breitkreutz I. Role of B-cell-activating factor in adhesion and growth of human multiple myeloma cells in the bone marrow microenvironment Cancer Res 2006; 66: 6675-82., 29Fu L, Lin-Lee YC, Pham LV. Constitutive NF-kappaB and NFAT activation leads to stimulation of the BLyS survival pathway in aggressive B-cell lymphomas Blood 2006; 107: 4540-8.], it is therefore likely that TLR engagement leads to BAFF transcriptional activation. The transcription factors regulating APRIL expression are less well known, but could also involve NF-κB. As regards BAFF-R, its enhanced expression following the engagement of TLR-7 in CLL cells was also dependent on the activation of NF-κB, since it was markedly impaired in the presence of wedelolactone, an inhibitor of IκBa phosphorylation. In addition, the stimulation of BAFF and APRIL expression elicited by Resiquimod appears to involve the activation of the AP-1 transcription factor and the phosphorylation of c-Jun. Furthermore, both the Resiquimod-driven protection toward apoptosis and the increased BAFF and APRIL expression were markedly inhibited in the presence of JNK Inhibitor II, a specific inhibitor of the phosphorylation of c-Jun. In agreement, it was previously reported that, in a human T cell line, the expression of BAFF is regulated through a transduction pathway involving the c-jun NH2-terminal kinase [30Yoshimoto K, Takahashi Y, Ogasawara M. Aberrant expression of BAFF in T cells of systemic lupus erythematosus, which is recapitulated by a human T cell line, Loucy Int Immunol 2006; 18: 1189-96.].
The up-regulation of BAFF, APRIL and BAFF-R resulting from TLR-7 engagement favors autocrine loops of survival. In turn, BAFF and APRIL can amplify the activation of NF-κB [17Kern C, Cornuel JF, Billard C. Involvement of BAFF and APRIL in the resistance to apoptosis of B-CLL through an autocrine pathway Blood 2004; 103: 679-88.] and eventually contribute to stimulate iNOS expression and NO production. Indeed, we observed that addition of exogenous BAFF or APRIL to ex vivo cultures of CLL cells triggers an increase in the production of NO by the leukemia cells, in about 25 % of the cases, notably those displaying a low spontaneous expression of iNOS (unpublished observations). In addition to their own pro-survival effects, BAFF and APRIL could amplify the biosynthesis of the anti-apoptotic agent NO when the production of the latter is not optimum.
The physiological ligand of TLR-7 has been identified as single strand RNA (ssRNA) [31Heil F, Hemmi H, Hochrein H. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8 Science 2004; 303: 1526-9., 32Diebold SS, Kaisho T, Hemmi H. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA Science 2004; 303: 1529-31.]. It is thereof likely that CLL cells or their precursors could be triggered through TLR-7 by ssRNA present in their microenvironment, either as microbial components (PAMPs, pathogen-associated molecular patterns) or as degradation products of self origin. This would provide to these cells, through the stimulation of the BAFF/APRIL pathways, an enhanced resistance toward spontaneous apoptosis favoring their accumulation.
It could also be suggested that a simultaneous co-ordinated stimulation of the BCR via these auto-antigens or microbial antigens would reinforce this resistance and contribute to the aetiology of this leukemia. This hypothesis is supported by the sequences of the monoclonal immunoglobulins of the BCR of CLL cells that, for many of them, present a bias suggestive of an encounter with type 2 T-independent antigens such as some auto-antigens and antigens of microbial origin. The role of TLR-7 is therefore compatible with the hypothesis of a chronic stimulation of B-lymphocytes or their precursors with a peculiar set of antigens (auto antigens or microbial-derived antigens) and PAMPs (pathogen-associated molecular patterns) in the onset of CLL.
BAFF is required for the development of mature B cell. Self-reactive B cells have reduced responsiveness to BAFF and die due to the limiting levels of BAFF available in vivo. Elevated BAFF expression provided by TLR-7 ligation rescue self-reactive B cells normally deleted late during maturation [33Brink R. Regulation of B cell self-tolerance by BAFF Semin Immunol 2006; 18: 276-83.]. This could explain the large proportion of sIg from both mutated and unmutated CLL patients displaying antibody activity against self components [6LanemoMyhrinder A, Hellqvist E, Sidorova E. A new perspective molecular motifs on oxidized LDL apoptotic cells and bacteria are targets for chronic lymphocytic leukemia antibodies Blood 2008; 111: 3838-48.].
BAFF-R is highly expressed on murine CD5+ B1 lymphocytes and the engagement of TLR-9 augments the surface expression of BAFF receptors and renders them responsive to BAFF co stimulation, mostly through BAFF-R up-regulation [34Ng LG, Ng CH, Woehl B. BAFF costimulation of Toll-like receptor-activated B-1 cells Eur J Immunol 2006; 36: 1837-46.]. In turn, BAFF stimulates CLL survival through activation of the canonical pathway of NF-κB activation [35Endo T, Nishio M, Enzler T. BAFF and APRIL support chronic lymphocytic leukemia B-cell survival through activation of the canonical NF-kappaB pathway Blood 2007; 109: 703-10.].
Attempts have been made to increase the immuno-genicity of CLL cells through stimulation of TLR-7 and/or TLR-9 in order to favor their elimination by the immune system [reviewed in 36]. In contrast to TLR-9-activated cells, however, TLR-7-activated CLL cells are weak stimulators of T cells proliferation and additional signals are required. The response of CLL cells to TLR agonists may also vary depending on their state of activation and the microenvironment: quiescent cells in the blood compartment versus proliferating cells in the bone marrow and the lymph nodes [37Caligaris-Cappio F. The microenvironment in CLL Br J Haematol 2003; 123: 380-8.]. Moreover, CLL cells activated by TLR-7 agonists become more sensitive to cycle-active cytotoxic drugs [38Goodman MG, Wormsley SB, Spinosa JC. Loxoribine induces CLL cells to traverse the cell cycle Blood 1994; 84: 3457-64.] and are also killed more easily by CTLs in vitro, although the mechanisms involved are not quite elucidated [39Spaner DE, Shi Y, Mena J. Immunomodulatory effects of TLR7 activation on CLL cells Leukemia 2006; 20: 286-95.].
In conclusion, our results demonstrate that the engagement of TLR-7 leads to increased resistance of CLL toward apoptosis due to a stimulation of the BAFF and APRIL survival pathways. They support the hypothesis of a chronic stimulation of TLR-7 by ssRNA of self or microorganism origine at the onset of the disease. They also indicate that the therapeutic attempts to increase the immunogenicity of CLL cells (or their sensitivity to cell cycle acting drugs) through stimulation with TLR agonists must take into consideration the risk of simultaneously stimulating their resistance to apoptosis. In the near future, we will try, in association with classical chemotherapy, to inhibit the BAFF and APRIL survival pathways, notably by preventing their stimulation through TLR.
ACKNOWLEDGEMENTS
This work was supported by INSERM, University Pierre and Marie Curie and by a grant from the Canceropôle Ile-de-France.